Levamisole (hydrochloride)
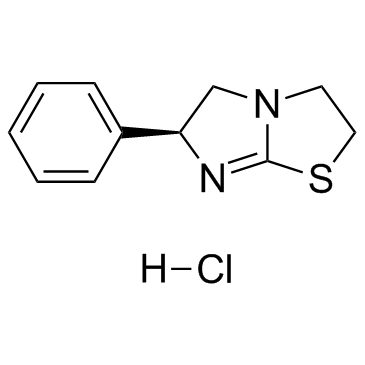
Levamisole (hydrochloride) structure
|
Common Name | Levamisole (hydrochloride) | ||
|---|---|---|---|---|
| CAS Number | 16595-80-5 | Molecular Weight | 240.752 | |
| Density | N/A | Boiling Point | 344.4ºC at 760 mmHg | |
| Molecular Formula | C11H13ClN2S | Melting Point | 226-231ºC | |
| MSDS | Chinese USA | Flash Point | 162.1ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Levamisole (hydrochloride)Levamisole Hcl is an anthelmintic and immunomodulator belonging to a class of synthetic imidazothiazole derivatives.IC50 value: Target: Levamisole suppresses the production of white blood cells, resulting in neutropenia and agranulocytosis. With the increasing use of levamisole as an adulterant, a number of these complications have been reported among cocaine users [1] [2]. Levamisole reversibly and noncompetitively inhibits most isoforms of alkaline phosphatase (e.g., human liver, bone, kidney, and spleen) except the intestinal and placental isoform [3]. It is thus used as an inhibitor along with substrate to reduce background alkaline phosphatase activity in biomedical assays involving detection signal amplification by intestinal alkaline phosphatase, for example in in situ hybridization or Western blot protocols. It is used to immobilize the nematode C. elegans on glass slides for imaging. |
| Name | Levamisole hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Levamisole Hcl is an anthelmintic and immunomodulator belonging to a class of synthetic imidazothiazole derivatives.IC50 value: Target: Levamisole suppresses the production of white blood cells, resulting in neutropenia and agranulocytosis. With the increasing use of levamisole as an adulterant, a number of these complications have been reported among cocaine users [1] [2]. Levamisole reversibly and noncompetitively inhibits most isoforms of alkaline phosphatase (e.g., human liver, bone, kidney, and spleen) except the intestinal and placental isoform [3]. It is thus used as an inhibitor along with substrate to reduce background alkaline phosphatase activity in biomedical assays involving detection signal amplification by intestinal alkaline phosphatase, for example in in situ hybridization or Western blot protocols. It is used to immobilize the nematode C. elegans on glass slides for imaging. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 344.4ºC at 760 mmHg |
|---|---|
| Melting Point | 226-231ºC |
| Molecular Formula | C11H13ClN2S |
| Molecular Weight | 240.752 |
| Flash Point | 162.1ºC |
| Exact Mass | 240.048798 |
| PSA | 40.90000 |
| LogP | 2.32160 |
| Index of Refraction | -126 ° (C=1, H2O) |
| Storage condition | 2~8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25 |
| Safety Phrases | S28-S45 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | NJ5960000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933290090 |
|
~% 
Levamisole (hyd... CAS#:16595-80-5 |
| Literature: Tetrahedron, , vol. 41, # 12 p. 2465 - 2470 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Polyoxometalates--potent and selective ecto-nucleotidase inhibitors.
Biochem. Pharmacol. 93(2) , 171-81, (2015) Polyoxometalates (POMs) are inorganic cluster metal complexes that possess versatile biological activities, including antibacterial, anticancer, antidiabetic, and antiviral effects. Their mechanisms o... |
|
|
Denervation impairs regeneration of amputated zebrafish fins.
BMC Dev. Biol. 14(1) , 49, (2015) Zebrafish are able to regenerate many of its tissues and organs after damage. In amphibians this process is regulated by nerve fibres present at the site of injury, which have been proposed to release... |
|
|
Leishmania amazonensis: characterization of an ecto-pyrophosphatase activity.
Exp. Parasitol. 137 , 8-13, (2014) Several ecto-enzymatic activities have been described in the plasma membrane of the protozoan Leishmania amazonensis, which is the major etiological agent of diffuse cutaneous leishmaniasis in South A... |
| (-)-Tetramisole hydrochloride |
| Solaskil |
| Imidazo[2,1-b]thiazole, 2,3,5,6-tetrahydro-6-phenyl-, (6S)-, hydrochloride (1:1) |
| Spartakon |
| Levamisol hydrochloride |
| (-)-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole hydrochloride |
| (6S)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazolhydrochlorid |
| (S)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole hydrochloride |
| Ascaridil |
| Levasole |
| Meglum |
| Levadin |
| MFCD00005536 |
| (S)-2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole Monohydrochloride |
| L[-]-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole |
| (-)-2,3,5,6-Tetrahydro-6-phenylimidazo(2,1-b)thiazole monohydrochloride |
| Decaris |
| L-(-)-2,3,5,6-Tetrahydro-6-phenyl-imidazo(2,1-b)thiazole hydrochloride |
| (-)-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole monohydrochloride |
| Nilverm |
| Ripercol |
| L(−)-2,3,5,6-Tetrahydro-6-phenylimidazo(2,1-b)thiazole hydrochloride |
| Levamisole HCl |
| Imidazo(2,1-b)thiazole, 2,3,5,6-tetrahydro-6-phenyl-, monohydrochloride, L-(-)- |
| L-(-)-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole Monohydrochloride |
| EINECS 240-654-6 |
| Tramisol |
| (6S)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazole hydrochloride (1:1) |
| L-Tetramisole hydrochloride |
| Levamisole hydrochloride |
| Imidazo[2,1-b]thiazole, 2,3,5,6-tetrahydro-6-phenyl-, monohydrochloride, L-(-)- |
| Levacide |
| Ergamisol |
| Nemicide |
| (6S)-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazole hydrochloride |
| Levamisole (hydrochloride) |
![Imidazo[2,1-b]thiazole,2,3,5,6-tetrahydro-6-phenyl- structure](https://image.chemsrc.com/caspic/466/5036-02-2.png)
![(R)-2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole monohydrochloride structure](https://image.chemsrc.com/caspic/460/16595-76-9.png)
 CAS#:14769-73-4
CAS#:14769-73-4
