Samuraciclib
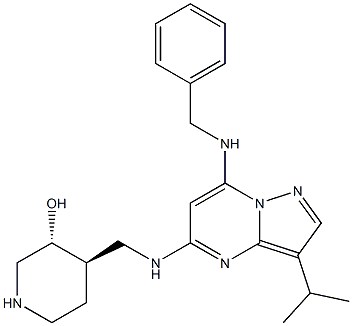
Samuraciclib structure
|
Common Name | Samuraciclib | ||
|---|---|---|---|---|
| CAS Number | 1805833-75-3 | Molecular Weight | 394.51 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C22H30N6O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of SamuraciclibSamuraciclib (CT7001) is a potent, selective, ATP-competitive and orally active CDK7 inhibitor, with an IC50 of 41 nM. Samuraciclib displays 45-, 15-, 230- and 30-fold selectivity over CDK1, CDK2 (IC50 of 578 nM), CDK5 and CDK9, respectively. Samuraciclib inhibits the growth of breast cancer cell lines with GI50 values between 0.2-0.3 µM. Samuraciclib has anti-tumor effects[1][2]. |
| Name | 3-Piperidinol, 4-[[[3-(1-methylethyl)-7-[(phenylmethyl)amino]pyrazolo[1,5-a]pyrimidin-5-yl]amino]methyl]-, (3R,4R)- |
|---|---|
| Synonym | More Synonyms |
| Description | Samuraciclib (CT7001) is a potent, selective, ATP-competitive and orally active CDK7 inhibitor, with an IC50 of 41 nM. Samuraciclib displays 45-, 15-, 230- and 30-fold selectivity over CDK1, CDK2 (IC50 of 578 nM), CDK5 and CDK9, respectively. Samuraciclib inhibits the growth of breast cancer cell lines with GI50 values between 0.2-0.3 µM. Samuraciclib has anti-tumor effects[1][2]. |
|---|---|
| Related Catalog | |
| Target |
CDK7/CycH/MAT1:41 nM (IC50) CDK2/cycE1:578 nM (IC50) CDK1:1.8 μM (IC50) CDK4:49 μM (IC50) CDK5:9.4 μM (IC50) CDK6:34 μM (IC50) CDK9:1.2 μM (IC50) |
| In Vitro | Samuraciclib (ICEC0942; 0-10 µM; 24 hours; HCT116 cells) treatment promotes cell apoptosis[1]. Samuraciclib (ICEC0942; 0-10 µM; 24 hours; HCT116 cells) treatment induces cell cycle arrest[1]. Samuraciclib (ICEC0942; 0-10 µM; 0-24 hours; HCT116 cells) treatment inhibits the phosphorylation of PolII CTD in a dose and time dependent manner in HCT116 colon cancer cells. Samuraciclib also inhibits phosphorylation of CDK1, CDK2 and retinoblastoma[1]. Samuraciclib (ICEC0942) inhibits the growth of MCF7, T47D, MDA-MB-231, HS578T, MDA-MB-468, MCF10A and HMEC cells with GI50 values of 0.18 µM, 0.32 µM, 0. 33 µM, 0.21 µM, 0.22 µM, 0.67 µM and 1.25 µM, respectively[1]. Apoptosis Analysis[1] Cell Line: HCT116 cells Concentration: 0 µM, 0.1 µM, 1 µM and 10 µM Incubation Time: 24 hours Result: Induced caspase 3/7 and demonstrated PARP cleavage. Cell Cycle Analysis[1] Cell Line: HCT116 cells Concentration: 0 µM, 0.01 µM, 0.1 µM, 1 µM and 10 µM Incubation Time: 24 hours Result: Showed accumulation of cells in G2/M. Western Blot Analysis[1] Cell Line: HCT116 cells Concentration: 0 µM, 0.1 µM, 1 µM and 10 µM Incubation Time: 0 hour, 4 hours, 8 hours, 16 hours or 24 hours Result: PolII CTD phosphorylation was inhibited in a dose and time dependent manner in HCT116 colon cancer cells. |
| In Vivo | Samuraciclib (ICEC0942; 100 mg/kg; oral gavage; daily; for 14 days; female nu/nu-BALB/c athymic nude mice) treatment inhibits tumor growth by 60% at day 14, and is accompanied by highly significant reductions in PolII Ser2 and Ser5 phosphorylation in PBMCs and in tumors[1]. The combination of Samuraciclib (ICEC0942) and ICI 47699 treatment shows complete growth arrest of estrogen receptor (ER)-positive tumor xenografts[1]. Animal Model: Female nu/nu-BALB/c athymic nude mice (7-week old) with MCF7 cells[1]. Dosage: 100 mg/kg Administration: Oral gavage; daily; for 14 days Result: At day 14, tumor growth was inhibited by 60%. |
| References |
| Molecular Formula | C22H30N6O |
|---|---|
| Molecular Weight | 394.51 |
| PPDA-001 |
| Samuraciclib |