Rosamultic acid
Modify Date: 2024-01-04 15:56:48
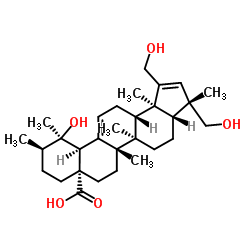
Rosamultic acid structure
|
Common Name | Rosamultic acid | ||
|---|---|---|---|---|
| CAS Number | 214285-76-4 | Molecular Weight | 486.683 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 630.2±55.0 °C at 760 mmHg | |
| Molecular Formula | C30H46O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 348.9±28.0 °C | |
Use of Rosamultic acidRosamultic acid is an A-ring contracted triterpene, that can be isolated from the roots of Rosa rnultiflora. Rosamultic acid inhibits gastric cancer cells proliferation by inducing Apoptosis mediated through cell cycle arrest, downregulation of cell cycle related protein expressions, inhibition of cell migration, DNA damage, and activation of caspases[1][2]. |
| Name | (3S,3aR,5aR,5bS,7aS,10R,11R,11aS,13aS,13bR)-11-Hydroxy-1,3-bis(hydroxymethyl)-3,5a,5b,10,11,13b-hexamethyl-3,3a,4,5,5a,5b,6,7,8,9,10,11,11a,13,13a,13b-hexadecahydro-7aH-cyclopenta[a]chrysene-7a-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Rosamultic acid is an A-ring contracted triterpene, that can be isolated from the roots of Rosa rnultiflora. Rosamultic acid inhibits gastric cancer cells proliferation by inducing Apoptosis mediated through cell cycle arrest, downregulation of cell cycle related protein expressions, inhibition of cell migration, DNA damage, and activation of caspases[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 630.2±55.0 °C at 760 mmHg |
| Molecular Formula | C30H46O5 |
| Molecular Weight | 486.683 |
| Flash Point | 348.9±28.0 °C |
| Exact Mass | 486.334534 |
| LogP | 5.87 |
| Vapour Pressure | 0.0±4.2 mmHg at 25°C |
| Index of Refraction | 1.593 |
| (3S,3aR,5aR,5bS,7aS,10R,11R,11aS,13aS,13bR)-11-Hydroxy-1,3-bis(hydroxymethyl)-3,5a,5b,10,11,13b-hexamethyl-3,3a,4,5,5a,5b,6,7,8,9,10,11,11a,13,13a,13b-hexadecahydro-7aH-cyclopenta[a]chrysene-7a-carboxylic acid |
| 7aH-Cyclopenta[a]chrysene-7a-carboxylic acid, 3,3a,4,5,5a,5b,6,7,8,9,10,11,11a,13,13a,13b-hexadecahydro-11-hydroxy-1,3-bis(hydroxymethyl)-3,5a,5b,10,11,13b-hexamethyl-, (3S,3aR,5aR,5bS,7aS,10R,11R,11aS,13aS,13bR)- |