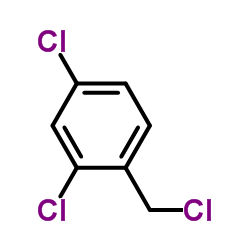
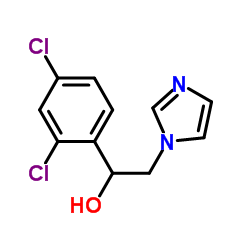
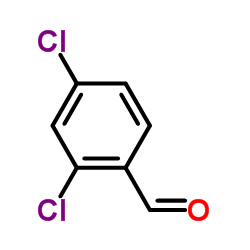
Miconazole
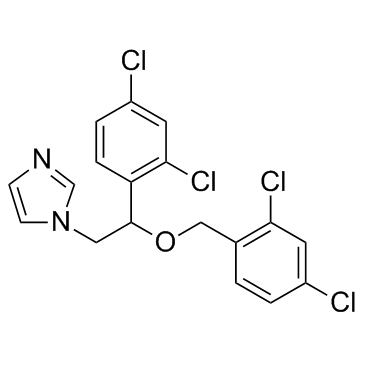
Miconazole structure
|
Common Name | Miconazole | ||
|---|---|---|---|---|
| CAS Number | 22916-47-8 | Molecular Weight | 416.129 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 555.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C18H14Cl4N2O | Melting Point | 159-163ºC | |
| MSDS | N/A | Flash Point | 289.5±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of MiconazoleMiconazole (Monistat) is an imidazole antifungal agent.Target: AntifungalMiconazole is an imidazole antifungal agent, developed by Janssen Pharmaceutica, commonly applied topically to the skin or to mucous membranes to cure fungal infections. It works by inhibiting the synthesis of ergosterol, a critical component of fungal cell membranes. It can also be used against certain species of Leishmania protozoa which are a type of unicellular parasite that also contain ergosterol in their cell membranes. In addition to its antifungal and antiparasitic actions, it also has some antibacterial properties. Miconazole is also used in Ektachrome film developing in the final rinse of the Kodak E-6 process and similar Fuji CR-56 process, replacing formaldehyde. Fuji Hunt also includes miconazole as a final rinse additive in their formulation of the C-41RA rapid access color negative developing process. From Wikipedia. |
| Name | Miconazole |
|---|---|
| Synonym | More Synonyms |
| Description | Miconazole (Monistat) is an imidazole antifungal agent.Target: AntifungalMiconazole is an imidazole antifungal agent, developed by Janssen Pharmaceutica, commonly applied topically to the skin or to mucous membranes to cure fungal infections. It works by inhibiting the synthesis of ergosterol, a critical component of fungal cell membranes. It can also be used against certain species of Leishmania protozoa which are a type of unicellular parasite that also contain ergosterol in their cell membranes. In addition to its antifungal and antiparasitic actions, it also has some antibacterial properties. Miconazole is also used in Ektachrome film developing in the final rinse of the Kodak E-6 process and similar Fuji CR-56 process, replacing formaldehyde. Fuji Hunt also includes miconazole as a final rinse additive in their formulation of the C-41RA rapid access color negative developing process. From Wikipedia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 555.1±50.0 °C at 760 mmHg |
| Melting Point | 159-163ºC |
| Molecular Formula | C18H14Cl4N2O |
| Molecular Weight | 416.129 |
| Flash Point | 289.5±30.1 °C |
| Exact Mass | 413.986023 |
| PSA | 27.05000 |
| LogP | 5.93 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.625 |
|
Material Safety Data Sheet
Section1. Identification of the substance Product Name: Miconazole Synonyms: Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed. H302:Harmful if swallowed
H317:May cause an allergic skin reaction P261:Avoid breathing dust/fume/gas/mist/vapours/spray P280:Wear protective gloves/protective clothing/eye protection/face protection P301+P312:IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell P363:Wash contaminated clothing before reuse P321:Specific treatment (see on this label) Section3. Composition/information on ingredients. Ingredient name:Miconazole CAS number:22916-47-8 Section4. First aid measures Skin contact:Immediately wash skin with copious amounts of water for at least 15 minutes while removing contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Inhalation:Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Ingestion:Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Storage:Store in closed vessels. Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Appearance:Not specified No data Boiling point: Melting point:No data Flash point:No data Density:No data Molecular formula: C18H14Cl4N2O Molecular weight: 416.1 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~70% 
Miconazole CAS#:22916-47-8 |
| Literature: Cruz-Almanza, Raymundo; Hernandez, Teresa; Perez, Francisco; Lemini, Cristina Organic Preparations and Procedures International, 1992 , vol. 24, # 3 p. 342 - 345 |
|
~% 
Miconazole CAS#:22916-47-8 |
| Literature: Organic Preparations and Procedures International, , vol. 24, # 3 p. 342 - 345 |
|
~% 
Miconazole CAS#:22916-47-8 |
| Literature: Organic Preparations and Procedures International, , vol. 24, # 3 p. 342 - 345 |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Simultaneous determination of seven azole antifungal drugs in serum by ultra-high pressure liquid chromatography and diode array detection.
Acta Clin. Belg. 69(1) , 53-61, (2014) Azole antifungals are a group of fungistatic agents that can be administered orally or parenterally. The determination of the concentrations of these antifungals (miconazole, fluconazole, ketoconazole... |
|
|
A comparison of in vitro ADME properties and pharmacokinetics of azithromycin and selected 15-membered ring macrolides in rodents.
Eur. J. Drug Metab. Pharmacokinet. 39(4) , 263-76, (2014) The purpose of this study was to evaluate the impact of structural modifications on the 15-membered macrolactone ring and/or substituents on the in vitro ADME properties and in vivo pharmacokinetic (P... |
|
|
HPLC and chemometric methods for the simultaneous determination of miconazole nitrate and nystatin.
J. Chromatogr. Sci. 50(10) , 855-61, (2012) High-performance liquid chromatography (HPLC) and chemometric methods were applied to the simultaneous determination of the two nonsteroidal antifungal drugs, miconazole (MIC) and nystatin (NYS). The ... |
| 1-[2-(2,4-Dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-1H-imidazole |
| EINECS 245-324-5 |
| 1-[2,4-Dichloro-b-[(2,4-dichlorobenzyl)oxy]phenethyl]imidazole |
| Daktarin IV |
| MFCD00216019 |
| MONISTAT IV |
| Minostate |
| 1-(2,4-dichloro-β-((2,4-dichlorobenzyl)oxy)phenethyl) imidazole |
| 1H-Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)- |
| Desenex |
| Miconozole |
| (±)-Miconazole |
| Monistat |
| mjr1762 |
| 1-[2-(2,4-dichlorophenyl)-2-{[(2,4-dichlorophenyl)methyl]oxy}ethyl]-1H-imidazole |
| 1-{2-[(2,4-Dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole |
| micronazol |
| 1H-Imidazole, 1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]- |
| Miconazole |
| Zeasorb-AF |