6-(γ,γ-Dimethylallylamino)purine
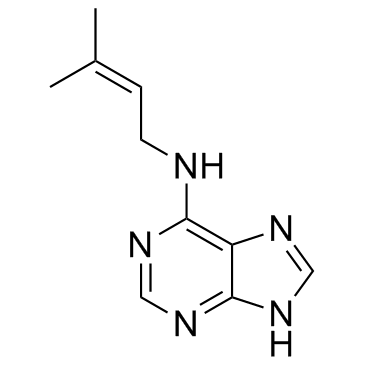
6-(γ,γ-Dimethylallylamino)purine structure
|
Common Name | 6-(γ,γ-Dimethylallylamino)purine | ||
|---|---|---|---|---|
| CAS Number | 2365-40-4 | Molecular Weight | 203.244 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 477.1±30.0 °C at 760 mmHg | |
| Molecular Formula | C10H13N5 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 242.4±24.6 °C | |
Use of 6-(γ,γ-Dimethylallylamino)purine6-(γ,γ-Dimethylallylamino)purine is a plant growth substance. |
| Name | N6-dimethylallyladenine |
|---|---|
| Synonym | More Synonyms |
| Description | 6-(γ,γ-Dimethylallylamino)purine is a plant growth substance. |
|---|---|
| Related Catalog | |
| In Vitro | Derivative UV spectroscopic data show that the plant growth substances 6-(γ,γ-Dimethylallylamino)purine (N6-(delta 2-isopentenyl) adenine, i6Ade)and indolylacetic acid (IAA) can bind to the yeast alcohol dehydrogenase (ADH) and affect coenzyme-enzyme binding. At fixed ethanol concentrations (27.8 and 111.1 mM) and varying NAD+ concentrations (0.033-2 mM), as well as at fixed levels of coenzyme (0.67 and 2 mM), and at varying concentrations of ethanol (1.4-111.1 mM), the rate of ethanol oxidation is significantly inhibited by i6Ade and IAA. The kinetics of the ADH reaction is affected by two inhibition constants (Ki and Ki') which correspond to the dissociation constants of complexes EI and ESI, respectively. For i6Ade the Ki=0.52±0.06 mM and Ki'=0.74±0.07 mM, and for IAA the Ki=0.88±0.03 mM and Ki'=0.99±0.02 mM[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 477.1±30.0 °C at 760 mmHg |
| Molecular Formula | C10H13N5 |
| Molecular Weight | 203.244 |
| Flash Point | 242.4±24.6 °C |
| Exact Mass | 203.117096 |
| PSA | 66.49000 |
| LogP | 2.31 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.683 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934993000 |
| Precursor 8 | |
|---|---|
| DownStream 5 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis, characterization and biological activity of ring-substituted 6-benzylamino-9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-ylpurine derivatives
Bioorg. Med. Chem. 17 , 1938-47, (2009) Synthesis of 9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-yl benzylaminopurines, their stability, cytokinin activity, perception by cytokinin receptors, degradation by cytokinin oxidase/dehydrogenas... |
|
|
Thidiazuron influences the endogenous levels of cytokinins and IAA during the flowering of isolated shoots of Dendrobium.
J. Plant Physiol. 163(11) , 1126-34, (2006) This study reports the effects of thidiazuron (TDZ) on the endogenous levels of indoleacetic acid (IAA), zeatin, zeatin riboside ([9R]Z), isopentenyladenine and isopentenyladenosine ([9R]iP) as well a... |
|
|
Functional identification of OsHk6 as a homotypic cytokinin receptor in rice with preferential affinity for iP.
Plant Cell Physiol. 53(7) , 1334-43, (2012) Cytokinins are involved in key developmental processes in rice (Oryza sativa), including the regulation of cell proliferation and grain yield. However, the in vivo action of histidine kinases (OsHks),... |
| 6-(y,y-Dimethylallylamino)purine |
| 6-isopentenyladenine |
| MFCD00132998 |
| N-(3-Methyl-2-buten-1-yl)-7H-purin-6-amine |
| N-(3-Methylbut-2-en-1-yl)-1H-purin-6-amin |
| iPeAde |
| 6-[(3-methylbut-2-en-1-yl)amino]-9H-purine |
| N-(3-methylbut-2-enyl)-7H-purin-6-amine |
| 6-[(3-Methyl-2-butenyl)amino]purine |
| N(6)-dimethylallyladenine |
| N6-[(3-methylbut-2-en-1-yl)amino]purine |
| N6-ISOPENTENYLADENINE (iP) |
| 3H-Purin-6-amine, N-(3-methyl-2-buten-1-yl)- |
| N6-(2-Isopentenyl)-adenosin |
| N-(3-methylbut-2-en-1-yl)-9H-purin-6-amine |
| N6-Isopentenyladenine |
| N6-(2-Isopentenyl)adenine |
| N6-(2-Isopentenyl)-adenine |
| N6-(δ 2-Isopentenyl)-adenine |
| Isopentenyl adenine |
| N6-iPeAde |
| adenine, isopentenyl- |
| N-(3-methylbut-2-en-1-yl)-1H-purin-6-amine |
| N6-(3-methylbut-2-enyl)adenine |
| isopentenyladenine |
| enadenine |
| TRIACANTHINE |
| N-Isopentenyladenine |
| 7H-Purin-6-amine, N-(3-methyl-2-buten-1-yl)- |
| N6-2-isopentenyladenine |
| Adenine, N- (3-methyl-2-butenyl)- |
| N-(3-methylbut-2-en-1-yl)-7H-purin-6-amine |
| N-(3-methylbut-2-enyl)adenine |
| Isopentenyl-Adenine |
| 2-iP N6-(2-Isopentenyl)adenine |
| 1H-Purin-6-amine, N- (3-methyl-2-butenyl)- |
| 2iP |
| N6-(delta 2-Isopentenyl)-adenine |
| N-(3-Methyl-2-buten-1-yl)-3H-purin-6-amine |
| N-(3-methyl-2-buten-1-yl)-1H-purin-6-amine |
| 6-(γ,γ-Dimethylallylamino)purine |
 CAS#:29911-54-4
CAS#:29911-54-4 CAS#:26728-58-5
CAS#:26728-58-5 CAS#:87-42-3
CAS#:87-42-3 CAS#:56329-06-7
CAS#:56329-06-7 CAS#:556-82-1
CAS#:556-82-1 CAS#:5451-40-1
CAS#:5451-40-1![[5-(6-acetamidopurin-9-yl)-3,4-diacetyloxy-oxolan-2-yl]methyl acetate Structure](https://image.chemsrc.com/caspic/032/7387-58-8.png) CAS#:7387-58-8
CAS#:7387-58-8 CAS#:7724-76-7
CAS#:7724-76-7 CAS#:6025-53-2
CAS#:6025-53-2 CAS#:13822-06-5
CAS#:13822-06-5 CAS#:68-94-0
CAS#:68-94-0![3H-Purine-3-butanoicacid, a-amino-6-[(3-methyl-2-buten-1-yl)amino]-,(aS)- structure](https://image.chemsrc.com/caspic/066/62061-49-8.png) CAS#:62061-49-8
CAS#:62061-49-8
