Homovanillyl alcohol
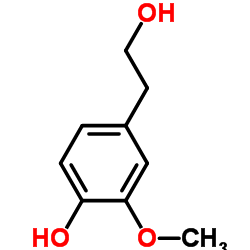
Homovanillyl alcohol structure
|
Common Name | Homovanillyl alcohol | ||
|---|---|---|---|---|
| CAS Number | 2380-78-1 | Molecular Weight | 168.190 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 316.8±27.0 °C at 760 mmHg | |
| Molecular Formula | C9H12O3 | Melting Point | 40-42 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 145.4±23.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Homovanillyl alcoholHomovanillyl alcohol is a biological metabolite of Hydroxytyrosol. Hydroxytyrosol is a phenolic compound that is present in virgin olive oil (VOO) and wine. Homovanillyl alcohol protects red blood cells (RBCs) from oxidative injury and has protective effect on cardiovascular disease[1][2]. |
| Name | 4-(2-hydroxyethyl)-2-methoxyphenol |
|---|---|
| Synonym | More Synonyms |
| Description | Homovanillyl alcohol is a biological metabolite of Hydroxytyrosol. Hydroxytyrosol is a phenolic compound that is present in virgin olive oil (VOO) and wine. Homovanillyl alcohol protects red blood cells (RBCs) from oxidative injury and has protective effect on cardiovascular disease[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 316.8±27.0 °C at 760 mmHg |
| Melting Point | 40-42 °C(lit.) |
| Molecular Formula | C9H12O3 |
| Molecular Weight | 168.190 |
| Flash Point | 145.4±23.7 °C |
| Exact Mass | 168.078644 |
| PSA | 49.69000 |
| LogP | 0.33 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.559 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2909499000 |
| Precursor 8 | |
|---|---|
| DownStream 3 | |
| HS Code | 2909499000 |
|---|---|
| Summary | 2909499000. ether-alcohols and their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Antagonistic control of a dual-input mammalian gene switch by food additives.
Nucleic Acids Res. 42(14) , e116, (2014) Synthetic biology has significantly advanced the design of mammalian trigger-inducible transgene-control devices that are able to programme complex cellular behaviour. Fruit-based benzoate derivatives... |
|
|
Verifying the botanical authenticity of commercial tannins through sugars and simple phenols profiles.
Food Chem. 206 , 274-83, (2016) Commercial tannins from several botanical sources and with different chemical and technological characteristics are used in the food and winemaking industries. Different ways to check their botanical ... |
|
|
Phenylpropanoid glycoside analogues: enzymatic synthesis, antioxidant activity and theoretical study of their free radical scavenger mechanism.
PLoS ONE 6(6) , e20115, (2011) Phenylpropanoid glycosides (PPGs) are natural compounds present in several medicinal plants that have high antioxidant power and diverse biological activities. Because of their low content in plants (... |
| MFCD00002903 |
| EINECS 219-175-1 |
| 3-METHOXY-4-HYDROXYPHENYLETHANOL |
| 2-(4-hydroxy-3-methoxyphenyl)ethanol |
| 3-methoxy-4-hydroxyphenethyl alcohol |
| 4-Hydroxy-3-methoxyphenylethyl alcohol |
| 4-Hydroxy-3-methoxyphenethyl alcohol |
| 4-(2-Hydroxyethyl)-2-methoxyphenol |
| 4-Hydroxy-3-methoxyphenethanol |
| Homovanillyl alcohol |
| Homovanillic alcohol |
| Benzeneethanol, 4-hydroxy-3-methoxy- |
| MOPET |
| Benzeneethanol,4-hydroxy-3-methoxy- |
 CAS#:97-53-0
CAS#:97-53-0 CAS#:64881-05-6
CAS#:64881-05-6 CAS#:5703-24-2
CAS#:5703-24-2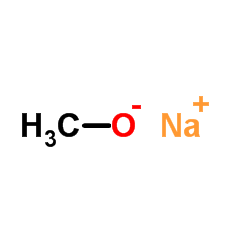 CAS#:124-41-4
CAS#:124-41-4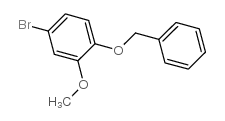 CAS#:63057-72-7
CAS#:63057-72-7 CAS#:1286729-19-8
CAS#:1286729-19-8 CAS#:67-56-1
CAS#:67-56-1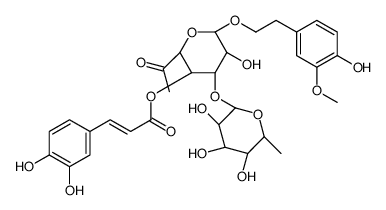 CAS#:94492-22-5
CAS#:94492-22-5 CAS#:10597-60-1
CAS#:10597-60-1