Vanillyl alcohol
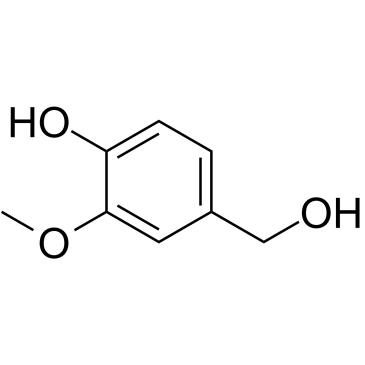
Vanillyl alcohol structure
|
Common Name | Vanillyl alcohol | ||
|---|---|---|---|---|
| CAS Number | 498-00-0 | Molecular Weight | 154.163 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 313.1±27.0 °C at 760 mmHg | |
| Molecular Formula | C8H10O3 | Melting Point | 110-117 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 143.2±23.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Vanillyl alcoholVanillyl alcohol (Vanillic alcohol), derived from vanillin, is a phenolic alcohol and is used as a flavoring agent in foods and beverages[1]. |
| Name | vanillyl alcohol |
|---|---|
| Synonym | More Synonyms |
| Description | Vanillyl alcohol (Vanillic alcohol), derived from vanillin, is a phenolic alcohol and is used as a flavoring agent in foods and beverages[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 313.1±27.0 °C at 760 mmHg |
| Melting Point | 110-117 °C(lit.) |
| Molecular Formula | C8H10O3 |
| Molecular Weight | 154.163 |
| Flash Point | 143.2±23.7 °C |
| Exact Mass | 154.062988 |
| PSA | 49.69000 |
| LogP | 0.00 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.570 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong acids. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29095090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 29095090 |
|---|
|
Synthesis and accumulation of aromatic aldehydes in an engineered strain of Escherichia coli.
J. Am. Chem. Soc. 136(33) , 11644-54, (2014) Aromatic aldehydes are useful in numerous applications, especially as flavors, fragrances, and pharmaceutical precursors. However, microbial synthesis of aldehydes is hindered by rapid, endogenous, an... |
|
|
High tolerance and physiological mechanism of Zymomonas mobilis to phenolic inhibitors in ethanol fermentation of corncob residue.
Biotechnol. Bioeng. 112 , 1770-82, (2015) Corncob residue as the lignocellulosic biomass accumulated phenolic compounds generated from xylitol production industry. For utilization of this biomass, Zymomonas mobilis ZM4 was tested as the ethan... |
|
|
Reactivity of phenolic compounds towards free radicals under in vitro conditions.
J. Food Sci. Technol. 52 , 5790-8, (2015) The free radical scavenging activity and reducing power of 16 phenolic compounds including four hydroxycinnamic acid derivatives namely ferulic acid, caffeic acid, sinapic acid and p-coumaric acid, be... |
| 4-Hydroxy-3-methoxybenzyl alcohol |
| Vanillyl alcohol |
| MFCD00004659 |
| Benzenemethanol, 4-hydroxy-3-methoxy- |
| 4-(Hydroxymethyl)-2-methoxyphenol |
| EINECS 207-852-4 |
 CAS#:121-33-5
CAS#:121-33-5 CAS#:121-34-6
CAS#:121-34-6 CAS#:93-51-6
CAS#:93-51-6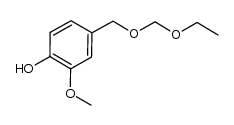 CAS#:1058649-10-7
CAS#:1058649-10-7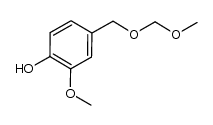 CAS#:1058649-06-1
CAS#:1058649-06-1 CAS#:86534-11-4
CAS#:86534-11-4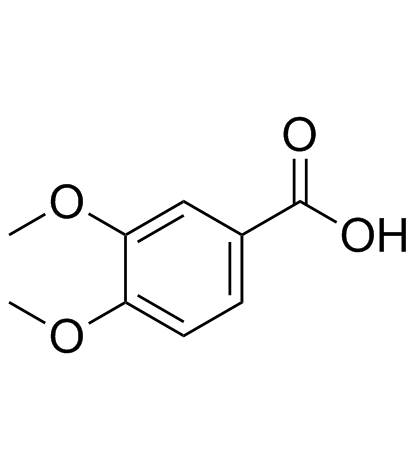 CAS#:93-07-2
CAS#:93-07-2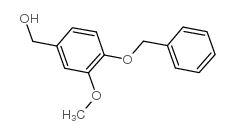 CAS#:33693-48-0
CAS#:33693-48-0 CAS#:495-76-1
CAS#:495-76-1 CAS#:109685-13-4
CAS#:109685-13-4 CAS#:1078-26-8
CAS#:1078-26-8 CAS#:3897-89-0
CAS#:3897-89-0 CAS#:3251-56-7
CAS#:3251-56-7 CAS#:6635-20-7
CAS#:6635-20-7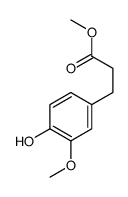 CAS#:56024-44-3
CAS#:56024-44-3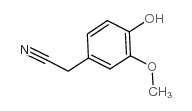 CAS#:4468-59-1
CAS#:4468-59-1![2-METHOXY-3-METHYL-[1,4]BENZOQUINONE structure](https://image.chemsrc.com/caspic/299/2207-57-0.png) CAS#:2207-57-0
CAS#:2207-57-0 CAS#:18102-32-4
CAS#:18102-32-4
