(4-Nitrophenyl)boronic acid
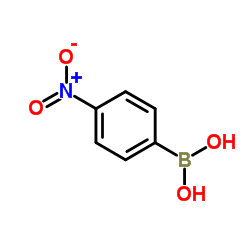
(4-Nitrophenyl)boronic acid structure
|
Common Name | (4-Nitrophenyl)boronic acid | ||
|---|---|---|---|---|
| CAS Number | 24067-17-2 | Molecular Weight | 166.93 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 373.7±44.0 °C at 760 mmHg | |
| Molecular Formula | C6H6BNO4 | Melting Point | 285-290°C (dec.) | |
| MSDS | USA | Flash Point | 179.8±28.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of (4-Nitrophenyl)boronic acid4-Nitrophenylboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | (4-nitrophenyl)boronic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Nitrophenylboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 4-Nitrophenylboronic acid 是一种芳基硼酸,已被用于合成苯酚。 |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 373.7±44.0 °C at 760 mmHg |
| Melting Point | 285-290°C (dec.) |
| Molecular Formula | C6H6BNO4 |
| Molecular Weight | 166.93 |
| Flash Point | 179.8±28.4 °C |
| Exact Mass | 167.038986 |
| PSA | 86.28000 |
| LogP | 1.32 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.574 |
| Storage condition | 2~8℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2931900090 |
|
~74% 
(4-Nitrophenyl)... CAS#:24067-17-2 |
| Literature: Angewandte Chemie - International Edition, , vol. 49, # 32 p. 5515 - 5518 |
|
~% 
(4-Nitrophenyl)... CAS#:24067-17-2 |
| Literature: Journal of the American Chemical Society, , vol. 134, # 28 p. 11667 - 11673 |
|
~0% 
(4-Nitrophenyl)... CAS#:24067-17-2 |
| Literature: Synlett, , # 1 art. no. G33904ST, p. 127 - 133 |
|
~% 
(4-Nitrophenyl)... CAS#:24067-17-2 |
| Literature: Synlett, , # 2 p. 266 - 268 |
|
~% 
(4-Nitrophenyl)... CAS#:24067-17-2 |
| Literature: Journal of the American Chemical Society, , vol. 134, # 28 p. 11667 - 11673 |
|
~% 
(4-Nitrophenyl)... CAS#:24067-17-2 |
| Literature: Journal of the American Chemical Society, , vol. 53, p. 713 |
|
~% 
(4-Nitrophenyl)... CAS#:24067-17-2 |
| Literature: Journal of Organic Chemistry, , vol. 73, # 12 p. 4662 - 4670 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic amines using arylboronates.
Org. Lett. 7th ed., 14 , 1930-1933, (2012) A Ru-catalyzed direct arylation of benzylic sp(3) carbons of acyclic amines with arylboronates is reported. This highly regioselective and efficient transformation can be performed with various combin... |
|
|
A new access to 3-substituted-1(2H)-isoquinolone by tandem palladium-catalyzed intramolecular aminocarbonylation annulation.
Org. Biomol. Chem. 13th ed., 10 , 2683-2691, (2012) An original tribromide derivative based, palladium-catalyzed synthesis of 3-substituted-1(2H)-isoquinolone is described based on a regioselective Suzuki-Miyaura C-C coupling on o-halo-(2,2-dihalovinyl... |
|
|
Copper-mediated sequential cyanation of aryl C-B and arene C-H bonds using ammonium iodide and DMF.
J. Am. Chem. Soc. 5th ed., 134 , 2528-2531, (2012) The cyanation of aromatic boronic acids, boronate esters, and borate salts was developed under copper-mediated oxidative conditions using ammonium iodide and DMF as the source of nitrogen and carbon a... |
| Phenylboronic Acid,9 |
| (4-Nitrophenyl)boronic acid |
| 4-Nitrobenzeneboronic acid p-Nitrophenylboronic acid p-nitro-benzeneboronic acid |
| para-nitrophenylboronic acid |
| phenylboronic acid pinacol ester |
| 4-Nitrobenzeneboronic Acid |
| MFCD00161360 |
| boronic acid,b-(4-nitrophenyl) |
| 4-Borononitrobenzene |
| Boronic acid, B-(4-nitrophenyl)- |
| p-nitrophenylboronic acid |
| 4-Nitrophenylboronic Acid |
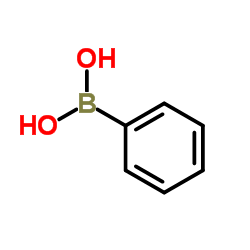
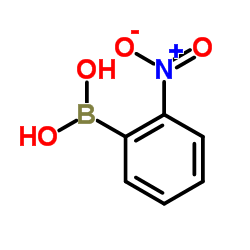

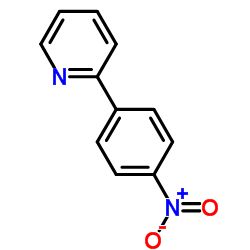 CAS#:4282-47-7
CAS#:4282-47-7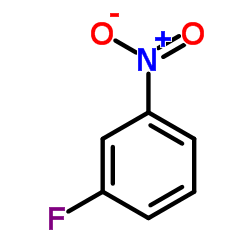 CAS#:402-67-5
CAS#:402-67-5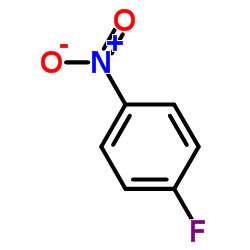 CAS#:350-46-9
CAS#:350-46-9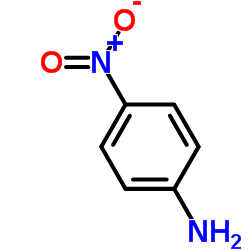 CAS#:100-01-6
CAS#:100-01-6 CAS#:92-93-3
CAS#:92-93-3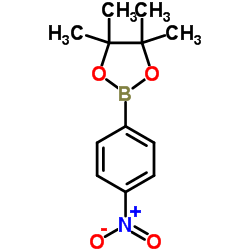 CAS#:171364-83-3
CAS#:171364-83-3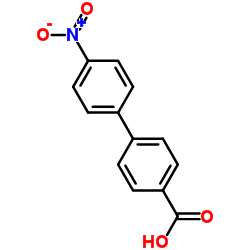 CAS#:92-89-7
CAS#:92-89-7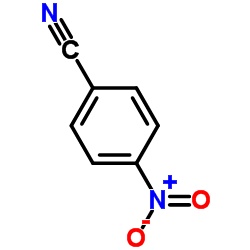 CAS#:619-72-7
CAS#:619-72-7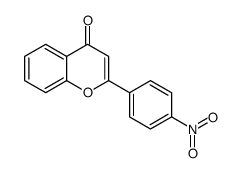 CAS#:19725-49-6
CAS#:19725-49-6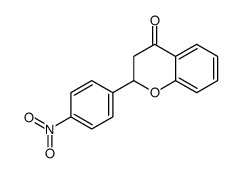 CAS#:3034-09-1
CAS#:3034-09-1
