5-Iodotubercidin
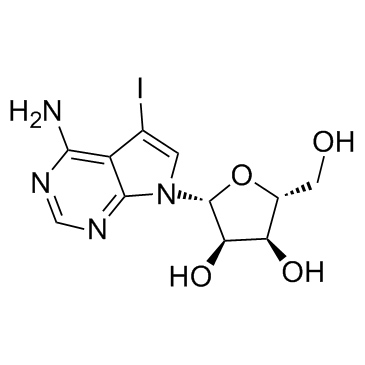
5-Iodotubercidin structure
|
Common Name | 5-Iodotubercidin | ||
|---|---|---|---|---|
| CAS Number | 24386-93-4 | Molecular Weight | 392.150 | |
| Density | 2.5±0.1 g/cm3 | Boiling Point | 701.5±60.0 °C at 760 mmHg | |
| Molecular Formula | C11H13IN4O4 | Melting Point | 216-217ºC dec. | |
| MSDS | Chinese USA | Flash Point | 378.0±32.9 °C | |
Use of 5-Iodotubercidin5-Iodotubercidin is a potent adenosine kinase inhibitor with IC50 of 26 nM. |
| Name | 5-iodotubercidin |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Iodotubercidin is a potent adenosine kinase inhibitor with IC50 of 26 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 26 nM (adenosine kinase) |
| In Vitro | 5-Iodotubercidin (40 μM) enhances the rate of phosphorylase inactivation and shortens the lag before the activation of glycogen synthase. 5-Iodotubercidin (50 μM) antagonizes the effects of glucagon and vasopressin, but does not affect the basal concentration of free calcium in single hepatocytes[1]. 5-Iodotubercidin (20 μM) causes an important decrease in ATP concentration, and a concomitant smaller increase in AMP concentration. 5-Iodotubercidin decreases the activity of ACC and the rates of synthesis of fatty acids and cholesterol. In line with the iodotubercidin-mediated inhibition of ACC, 5-iodotubercidin induces a marked decrease in the intracellular concentration of malonyl-CoA[3]. 5-Iodotubercidin causes a strong decrease in the immunofluorescence levels of P-T3-H3, and depletion of P-T3-H3 is complete at 10 µM 5-5-iodotubercidin[4]. |
| In Vivo | 5-Iodotubercidin (1 mL/kg, i.p.) is in agreement with activity observed against bicuculline-induced seizures following local administration of the AKI into the prepiriform cortex[2]. |
| Kinase Assay | AK activity is measured in a radiochemical assay. The final reaction volume is 100 µL and contained 70 mM Tris-maleate (pH 7.0), 0.1% (w/v) bovine serum albumin, 1.0 mM MgCl2, 1.0 mM ATP, 1.0 µM [U-14C]adenosine (400-600 mCi/mmol) and various inhibitor concentrations. Inhibitors are prepared as 10 mM stock solutions in DMSO. The final DMSO concentration in the assay is 5% (v/v). Eleven different concentration of the test solutions ranging from 0.001 to 10.0 µM are utilized to determine a dose response curve of the inhibition of the enzyme. Reactions are started by adding the appropriate amount of purified human recombinant AK and incubated for 20 min at 37°C. The reactions are terminated by addition of the potent AKI GP3269. A 30-µL aliquot of each reaction is spotted on DEAE cellulose filter paper (cut in squares of appr 1×1 cm) and air-dried for 30 min. The dry filters are then washed for 3 min in deionized water to remove residual [U-14C]adenosine, rinsed with ethanol and dried at 90°C for 20 min. The filter papers are counted in 5.5 mL of Ready Safe liquid scintillation cocktail using a Beckman LS3801 scintillation counter. Control AK activity is determined from the amount of [14C]AMP formed in the presence of 5% DMSO. The concentration of inhibitor required to inhibit 50% of the AK activity (IC50) is determined graphically from plots of inhibitor concentration versus percent (%) control enzyme activity. |
| Cell Assay | HeLa cells are grown in DME supplemented with 10% fetal bovine serum (FBS) and 2 mM l-glutamine. Nocodazole is used at a concentration of 3.3 µM unless differently specified. Thymidine (2.5 mM) is used in the asssay. For transfection, FuGENE 6 Transfection Agent is used at a 3:1 ratio with plasmid DNA. Cells are analyzed 24-48 h after transfection. |
| Animal Admin | Male SA rats (100-150 g) are maintained on a 12:12 light:dark cycle in temperaturecontrolled facilities with free access to food and water. One hour prior to seizure testing, the animals are injected intraperitoneally (1 mL/kg) with DMSO vehicle or with test compound dissolved in DMSO. At the time of the test, an electrolyte solution (2% lidocaine in 0.9% sodium chloride) is applied to the eyes. Maximal electroshock seizures are induced by administering a 60-Hz current of 150 mA for 0.2 s via corneal electrodes, using a Wahlquist Model H stimulator. The endpoint measured is suppression of hindlimb tonic extension (HTE) and expressed as percentage of animals in which the response is inhibited. At this supramaximal stimulation level, virtually 100% of control (vehicle-treated) animals show HTE. ED50 values are calculated from a dose-response curve using probit analysis. The N for the screening doses is 6-8; doseresponse determinations are conducted with at least 5 animals/dose. |
| References |
| Density | 2.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 701.5±60.0 °C at 760 mmHg |
| Melting Point | 216-217ºC dec. |
| Molecular Formula | C11H13IN4O4 |
| Molecular Weight | 392.150 |
| Flash Point | 378.0±32.9 °C |
| Exact Mass | 391.998138 |
| PSA | 126.65000 |
| LogP | 0.91 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.919 |
| Storage condition | 2-8°C |
| Stability | Store tightly sealed at 4°C; Light Sensitive |
| Water Solubility | 0.1 M HCl: 0.7 mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934999090 |
|
~72% 
5-Iodotubercidin CAS#:24386-93-4 |
| Literature: Seela, Frank; Ming, Xin Tetrahedron, 2007 , vol. 63, # 39 p. 9850 - 9861 |
|
~90% 
5-Iodotubercidin CAS#:24386-93-4 |
| Literature: Zhang, Liangren; Zhang, Yunlong; Li, Xianghui; Zhang, Lihe Bioorganic and Medicinal Chemistry, 2002 , vol. 10, # 4 p. 907 - 912 |
|
~% 
5-Iodotubercidin CAS#:24386-93-4 |
| Literature: Tetrahedron, , vol. 63, # 39 p. 9850 - 9861 |
|
~% 
5-Iodotubercidin CAS#:24386-93-4 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 10, # 4 p. 907 - 912 |
|
~% 
5-Iodotubercidin CAS#:24386-93-4 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 10, # 4 p. 907 - 912 |
|
~% 
5-Iodotubercidin CAS#:24386-93-4 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 10, # 4 p. 907 - 912 |
|
~% 
5-Iodotubercidin CAS#:24386-93-4 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 10, # 4 p. 907 - 912 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Chaperoning of the A1-adenosine receptor by endogenous adenosine - an extension of the retaliatory metabolite concept.
Mol. Pharmacol. 87(1) , 39-51, (2015) Cell-permeable orthosteric ligands can assist folding of G protein-coupled receptors in the endoplasmic reticulum (ER); this pharmacochaperoning translates into increased cell surface levels of recept... |
|
|
Endothelial and Neuronal Nitric Oxide Activate Distinct Pathways on Sympathetic Neurotransmission in Rat Tail and Mesenteric Arteries.
PLoS ONE 10 , e0129224, (2015) Nitric oxide (NO) seems to contribute to vascular homeostasis regulating neurotransmission. This work aimed at assessing the influence of NO from different sources and respective intracellular pathway... |
|
|
An adenosine kinase inhibitor, ABT-702, inhibits spinal nociceptive transmission by adenosine release via equilibrative nucleoside transporters in rat.
Neuropharmacology 97 , 160-70, (2015) Adenosine kinase (AK) inhibitor is a potential candidate for controlling pain, but some AK inhibitors have problems of adverse effects such as motor impairment. ABT-702, a non-nucleoside AK inhibitor,... |
| 7H-Pyrrolo[2,3-d]pyrimidin-4-amine, 5-iodo-7-β-D-ribofuranosyl- |
| MFCD00055131 |
| 5-Iodo-7-(β-D-ribofuranosyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine |
| 5-Iodotubercidin |
| 5-Iodotubericidin |
![(2R,3R,4R,5R)-2-((Benzoyloxy)Methyl)-5-(4-Chloro-5-Iodo-7H-Pyrrolo[2,3-D]Pyrimidin-7-Yl)Tetrahydrofuran-3,4-Diyl Dibenzoate structure](https://image.chemsrc.com/caspic/101/480439-89-2.png)
![7H-Pyrrolo[2,3-d]pyrimidine,4-chloro-5-iodo-7-b-D-ribofuranosyl- structure](https://image.chemsrc.com/caspic/247/24386-91-2.png)
![4-Chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine structure](https://image.chemsrc.com/caspic/393/123148-78-7.png)

![4-chloro-5-iodo-7-[5-O-[(1,1-dimethylethyl)dimethysilyl]-2,3-O-(1-methylethylidene)-β-D-ribofuranosyl]-7H-pyrrolo[2,3-d]pyrimidine structure](https://image.chemsrc.com/caspic/165/291535-38-1.png)
![(3aR,6R,6aR)-6-(((tert-Butyldimethylsilyl)oxy)methyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-ol structure](https://image.chemsrc.com/caspic/075/217309-46-1.png)
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine structure](https://image.chemsrc.com/caspic/189/3680-69-1.png)

