Nimbolide
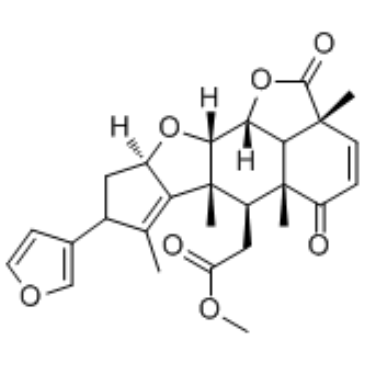
Nimbolide structure
|
Common Name | Nimbolide | ||
|---|---|---|---|---|
| CAS Number | 25990-37-8 | Molecular Weight | 466.52 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 608.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C27H30O7 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 321.9±31.5 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of NimbolideNimbolide is a triterpene derived from the leaves and flowers of neem (Azadirachta indica L). Nimbolide induces apoptosis through inactivation of NF-κB. Nimbolide inhibits CDK4/CDK6 kinase activity. Nimbolide suppresses the NF-κB, Wnt, PI3K-Akt, MAPK and JAK-STAT signaling pathways[1]. |
| Name | Nimbolide |
|---|---|
| Synonym | More Synonyms |
| Description | Nimbolide is a triterpene derived from the leaves and flowers of neem (Azadirachta indica L). Nimbolide induces apoptosis through inactivation of NF-κB. Nimbolide inhibits CDK4/CDK6 kinase activity. Nimbolide suppresses the NF-κB, Wnt, PI3K-Akt, MAPK and JAK-STAT signaling pathways[1]. |
|---|---|
| Related Catalog | |
| Target |
NF-κB CDK4 CDK6 |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 608.6±55.0 °C at 760 mmHg |
| Molecular Formula | C27H30O7 |
| Molecular Weight | 466.52 |
| Flash Point | 321.9±31.5 °C |
| Exact Mass | 466.199158 |
| PSA | 92.04000 |
| LogP | 2.66 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.597 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| RTECS | GY2370000 |
|
Nimbolide Induces ROS-Regulated Apoptosis and Inhibits Cell Migration in Osteosarcoma.
Int. J. Mol. Sci. 16 , 23405-24, (2015) Osteosarcoma (OS) is a primary malignant tumor of bone and is most prevalent in children and adolescents. OS is frequently associated with pulmonary metastasis, which is the main cause of OS-related m... |
|
|
Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture.
Southeast Asian J. Trop. Med. Public Health 16(1) , 66-72, (1985) The terpenoid lactone nimbolide, the structure of which has been unambiguously established, was found to inhibit Plasmodium falciparum in culture with a moderate potency. The EC50 against the parasite... |
|
|
Induction of cell cycle arrest, DNA damage, and apoptosis by nimbolide in human renal cell carcinoma cells.
Tumour Biol. 36(10) , 7539-47, (2015) Nimbolide is a tetranortriterpenoid isolated from the leaves and flowers of Azadirachta indica which has been shown to exhibit anticancer, antioxidant, anti-inflammatory, and anti-invasive properties ... |
| Methyl [8-(3-furyl)-2a,5a,6a,7-tetramethyl-2,5-dioxo-2a,5a,6,6a,8,9,9a,10a,10b,10c-decahydro-2H,5H-cyclopenta[b]furo[2',3',4':4,5]naphtho[2,3-d]furan-6-yl]acetate |
| 2H,5H-Cyclopenta[d]naphtho[2,3-b:1,8-b'c']difuran-6-acetic acid, 8-(3-furanyl)-2a,5a,6,6a,8,9,9a,10a,10b,10c-decahydro-2a,5a,6a,7-tetramethyl-2,5-dioxo-, methyl ester |
| (4S,4aS,8aS)-4-(tert-Butyl-dimethylsilyl)oxy-7,7-dimethoxy-4a-methyl-3,4,5,6,8,8a-hexahydro-2H-naphthalen-1-one |
| (4S,4aS,8aS)-4-(dimethyl-tert-butyl-silyl)oxy-7,7-dimethoxy-4a-methyl-decalin-1-one |