H-L-Phe(4-NH-Poc)-OH hydrochloride
Modify Date: 2024-04-03 12:01:57
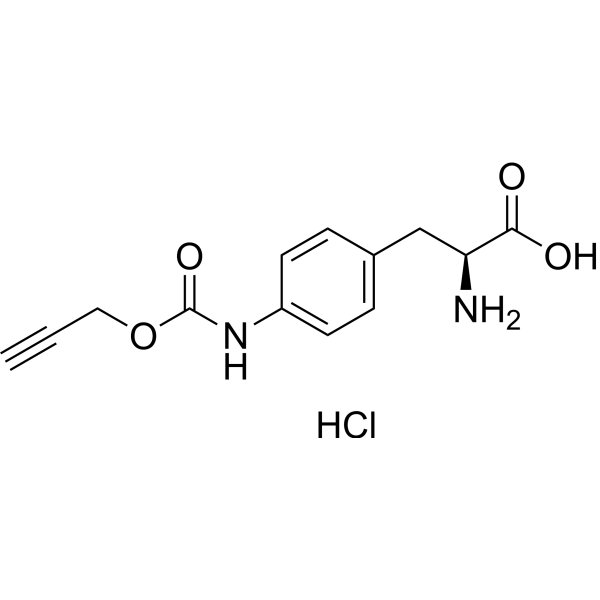
H-L-Phe(4-NH-Poc)-OH hydrochloride structure
|
Common Name | H-L-Phe(4-NH-Poc)-OH hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 2737202-66-1 | Molecular Weight | 298.72 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C13H15ClN2O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of H-L-Phe(4-NH-Poc)-OH hydrochlorideH-L-Phe(4-NH-Poc)-OH (hydrochloride) is a click chemistry reagent containing an azide group. Used as a modified Phe or Tyr analogue in protein and peptide biosynthesis. Propargyloxycarbonyl, commonly abbreviated as Poc or Pryoc, can either be used as alkyne component for standard Click conjugation or in combination with tetrazine linkers in copper-free Diels-Alder type Click reactions. It also has applications as unusual protecting group for amines, hydroxy functions and as esters. All 3 are stable to neat TFA, but can be cleaved at ambient temperature with Co2(CO)8 in TFA:DCM. Deprotection with other transition metals like palladium have also been reported[1][2]. |
| Name | H-L-Phe(4-NH-Poc)-OH hydrochloride |
|---|
| Description | H-L-Phe(4-NH-Poc)-OH (hydrochloride) is a click chemistry reagent containing an azide group. Used as a modified Phe or Tyr analogue in protein and peptide biosynthesis. Propargyloxycarbonyl, commonly abbreviated as Poc or Pryoc, can either be used as alkyne component for standard Click conjugation or in combination with tetrazine linkers in copper-free Diels-Alder type Click reactions. It also has applications as unusual protecting group for amines, hydroxy functions and as esters. All 3 are stable to neat TFA, but can be cleaved at ambient temperature with Co2(CO)8 in TFA:DCM. Deprotection with other transition metals like palladium have also been reported[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C13H15ClN2O4 |
|---|---|
| Molecular Weight | 298.72 |