L-Ornithine L-aspartate salt
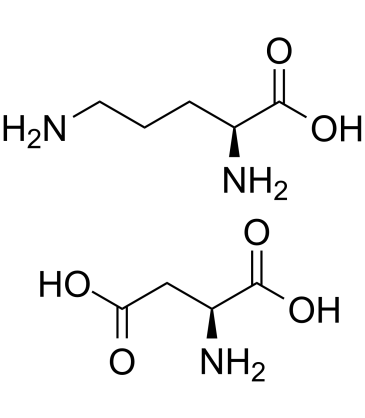
L-Ornithine L-aspartate salt structure
|
Common Name | L-Ornithine L-aspartate salt | ||
|---|---|---|---|---|
| CAS Number | 3230-94-2 | Molecular Weight | 265.264 | |
| Density | N/A | Boiling Point | 308.7ºC at 760 mmHg | |
| Molecular Formula | C9H19N3O6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 140.5ºC | |
Use of L-Ornithine L-aspartate saltL-Ornithine L-aspartate is a stable salt of two natural nonessential L-amino acids: ornithine and aspartic acid. L-Ornithine L-aspartate lowers blood ammonia concentration and to eliminate symptoms of hepatic encephalopathy associated with liver cirrhosis[1]. |
| Name | (2S)-2-aminobutanedioic acid,(2S)-2,5-diaminopentanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | L-Ornithine L-aspartate is a stable salt of two natural nonessential L-amino acids: ornithine and aspartic acid. L-Ornithine L-aspartate lowers blood ammonia concentration and to eliminate symptoms of hepatic encephalopathy associated with liver cirrhosis[1]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 308.7ºC at 760 mmHg |
|---|---|
| Molecular Formula | C9H19N3O6 |
| Molecular Weight | 265.264 |
| Flash Point | 140.5ºC |
| Exact Mass | 265.127380 |
| PSA | 189.96000 |
| LogP | 0.11110 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | CI9463000 |
| HS Code | 2922499990 |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
Modulation of neural activation following treatment of hepatic encephalopathy.
Neurology 80(11) , 1041-7, (2013) To measure changes in psychometric state, neural activation, brain volume (BV), and cerebral metabolite concentrations during treatment of minimal hepatic encephalopathy.As proof of principle, 22 pati... |
|
|
L-Ornithine-l-aspartate in the management of hepatic encephalopathy: a meta-analysis.
J. Gastroenterol. Hepatol. 24(1) , 9-14, (2009) Hepatic encephalopathy continues to be a major clinical problem and the current decade has not witnessed major therapeutic breakthroughs in this area. L-ornithine-l-aspartate (LOLA) is not frequently ... |
|
|
A double-blind, randomized, placebo-controlled trial of intravenous L-ornithine-L-aspartate on postural control in patients with cirrhosis.
Liver Int. 30(4) , 574-82, (2010) Hepatic encephalopathy (HE) is a complication of liver disease. Several treatments have been introduced but only L-ornithine-L-aspartate (LOLA) shows proven efficacy. This double-blind, randomized, pl... |
| L-Ornithine L-Aspartate Salt |
| L-Ornithine L-Aspartate |
| L-Ornithine-L-Aspartate |
| L-Aspartic acid - L-ornithine (1:1) |
| EINECS 221-772-7 |
| L-OrnithineL-aspartatesalt |
| (S)-2,5-Diaminopentanoic acid L-aspartate salt |
| Hepa-Merz (TN) |
| L-Ornithin-L-aspartat |
| L-Aspartic acid, compd. with L-ornithine (1:1) |
| MFCD00058084 |
| (S)-2,5-Diaminopentanoic acid compound with (S)-2-aminosuccinic acid |

