Ketotifen fumarate
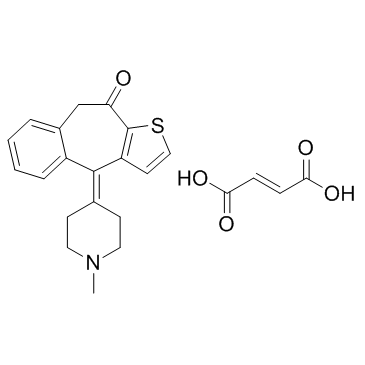
Ketotifen fumarate structure
|
Common Name | Ketotifen fumarate | ||
|---|---|---|---|---|
| CAS Number | 34580-14-8 | Molecular Weight | 425.497 | |
| Density | 0.968 g/mL at 25 °C(lit.) | Boiling Point | 250-263 °C(lit.) | |
| Molecular Formula | C23H23NO5S | Melting Point | -43°C | |
| MSDS | Chinese USA | Flash Point | 96-98°C/5mm | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Ketotifen fumarateKetotifen (fumarate) is a second-generation noncompetitive H1-antihistamine and mast cell stabilizer, which is used to prevent asthma attacks.Target: Histamine Receptor Ketotifen is a second-generation noncompetitive H1-antihistamine and mast cell stabilizer. It is most commonly sold as a salt of fumaric acid,ketotifen fumarate, and is available in two forms. In its ophthalmic form, it is used to treat allergic conjunctivitis, or the itchy red eyes caused by allergies. In its oral form, it is used to prevent asthma attacks. Side effects include drowsiness, weight gain, dry mouth, irritability, and increased nosebleeds.Ketotifen relieves and prevents eye itchiness and/or irritation associated with most seasonal allergies. It starts working within minutes after administering the drops. The drug has not been studied in children under three. The mean elimination half life is 12 hours. Besides its anti-histaminic activity, it is also a functional leukotriene antagonist and a phosphodiesterase inhibitor. The drug may also help relieve the symptoms of Irritable bowel syndrome. |
| Name | (E)-but-2-enedioic acid,10-(1-methylpiperidin-4-ylidene)-5H-benzo[1,2]cyclohepta[3,4-b]thiophen-4-one |
|---|---|
| Synonym | More Synonyms |
| Description | Ketotifen (fumarate) is a second-generation noncompetitive H1-antihistamine and mast cell stabilizer, which is used to prevent asthma attacks.Target: Histamine Receptor Ketotifen is a second-generation noncompetitive H1-antihistamine and mast cell stabilizer. It is most commonly sold as a salt of fumaric acid,ketotifen fumarate, and is available in two forms. In its ophthalmic form, it is used to treat allergic conjunctivitis, or the itchy red eyes caused by allergies. In its oral form, it is used to prevent asthma attacks. Side effects include drowsiness, weight gain, dry mouth, irritability, and increased nosebleeds.Ketotifen relieves and prevents eye itchiness and/or irritation associated with most seasonal allergies. It starts working within minutes after administering the drops. The drug has not been studied in children under three. The mean elimination half life is 12 hours. Besides its anti-histaminic activity, it is also a functional leukotriene antagonist and a phosphodiesterase inhibitor. The drug may also help relieve the symptoms of Irritable bowel syndrome. |
|---|---|
| Related Catalog | |
| References |
| Density | 0.968 g/mL at 25 °C(lit.) |
|---|---|
| Boiling Point | 250-263 °C(lit.) |
| Melting Point | -43°C |
| Molecular Formula | C23H23NO5S |
| Molecular Weight | 425.497 |
| Flash Point | 96-98°C/5mm |
| Exact Mass | 425.129700 |
| PSA | 123.15000 |
| LogP | 3.66410 |
| Index of Refraction | n20/D 1.522(lit.) |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DE8260000 |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Development and validation of an ultra-performance liquid chromatography method for simultaneous analysis of 20 antihistaminics in dietary supplements.
Biomed. Chromatogr. 29(3) , 465-74, (2015) The purpose of this study was to develop and validate an ultra-performance liquid chromatography method for simultaneous analysis of 20 antihistamines (illegal additives) in dietary supplements. The l... |
|
|
[Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options].
Dtsch. Med. Wochenschr. 139(30) , 1523-34; quiz 1535-8, (2014) In the present paper clinical phenotypes, pathogenetic relationships, and diagnostic algorithms as well as therapeutic concepts of/for systemic mast cell activation disease are reviewed. The reader sh... |
|
|
The effect of antihistamines on seizures induced by increasing-current electroshocks: ketotifen, but not olopatadine, promotes the seizures in infant rats.
Biol. Pharm. Bull. 35(5) , 693-7, (2012) Clinical reports have shown that some antihistamines, such as ketotifen, occasionally produced seizures, especially in pre-school age children or young patients with epilepsy. The purpose of this stud... |
| Ketotifen hydrogen fumarate |
| Zaditor |
| 4-(1-methylpiperidin-4-ylidene)-4,9-dihydro-10H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-one (2E)-but-2-enedioate |
| acide (2E)-but-2-ènedioïque - 4-(1-méthylpipéridin-4-ylidène)-4,9-dihydro-10H-benzo[4,5]cyclohepta[1,2-b]thiophén-10-one (1:1) |
| Ketotifen fumarate |
| 4-(1-Methylpiperidin-4-ylidene)-4,9-dihydro-10H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-one (2E)-but-2-enedioate (1:1) |
| UNII:HBD503WORO |
| Ketotifen fumarate salt |
| Alaway |
| 10H-Benzo[4,5]cyclohepta[1,2-b]thiophen-10-one, 4,9-dihydro-4-(1-methyl-4-piperidinylidene)-, (2E)-2-butenedioate (1:1) |
| (2E)-But-2-endisäure--4-(1-methylpiperidin-4-yliden)-4,9-dihydro-10H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-on(1:1) |
| Zaditen |
| UNII-HBD503WORO |
| MFCD00079394 |
| 4-(1-Methyl-4-piperidinylidene)-4,9-dihydro-10H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-one (2E)-2-butenedioate (1:1) |
| EINECS 252-100-0 |
| Ketotifen (fumarate) |

