Norgestimate
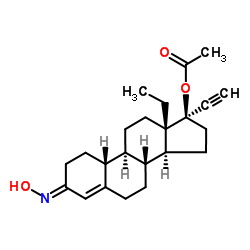
Norgestimate structure
|
Common Name | Norgestimate | ||
|---|---|---|---|---|
| CAS Number | 35189-28-7 | Molecular Weight | 369.497 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 497.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C23H31NO3 | Melting Point | 216ºC | |
| MSDS | Chinese USA | Flash Point | 254.9±28.7 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of NorgestimateNorgestimate, a synthetic progesterone analog, is an orally active progestin with highly selective progestational activity and minimal androgenicity. Norgestimate is used for an oral contraceptive[1][2]. |
| Name | norgestimate |
|---|---|
| Synonym | More Synonyms |
| Description | Norgestimate, a synthetic progesterone analog, is an orally active progestin with highly selective progestational activity and minimal androgenicity. Norgestimate is used for an oral contraceptive[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Norgestimate is converted to at least two active metabolites: levonorgestrel 3-oxime (deacetylated norgestimate; norelgestromin) and levonorgestrel[1]. |
| References |
[1]. Stanczyk FZ, et al. All progestins are not created equal. Steroids. 2003 Nov;68(10-13):879-90. |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 497.9±45.0 °C at 760 mmHg |
| Melting Point | 216ºC |
| Molecular Formula | C23H31NO3 |
| Molecular Weight | 369.497 |
| Flash Point | 254.9±28.7 °C |
| Exact Mass | 369.230408 |
| PSA | 58.89000 |
| LogP | 5.00 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.611 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H361fd |
| Precautionary Statements | P281-P305 + P351 + P338 |
| Hazard Codes | Xn |
| Risk Phrases | 63-22-36/38-62 |
| Safety Phrases | 26-36/37 |
| RIDADR | NONH for all modes of transport |
| RTECS | JF7976000 |
|
~85% 
Norgestimate CAS#:35189-28-7 |
| Literature: RICHTER GEDEON VEGYESZETI GYAR R.T. Patent: WO2005/868 A1, 2005 ; Location in patent: Page/Page column 5 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
Types of combined oral contraceptives used by US women.
Contraception 86(6) , 659-65, (2012) We sought to estimate the prevalence of types of combined oral contraceptives (COCs) used among US women.We analyzed interview-collected data from 12,279 women aged 15-44 years participating in the Na... |
|
|
Mechanosensitive ATP release from hemichannels and Ca²⁺ influx through TRPC6 accelerate wound closure in keratinocytes.
J. Cell Sci. 127(Pt 19) , 4159-71, (2014) The study aimed to investigate the effect of inhibition of poly(ADP-ribose) polymerase-1 (PARP-1) activity on tau phosphorylation in HEK293/tau441 cells and its mechanism. HEK293/tau441 cells were tre... |
|
|
Progestins in preventive hormone therapy. Including pharmacology of the new progestins, desogestrel, norgestimate, and gestodene: are there advantages?
Obstet. Gynecol. Clin. North Am. 21(2) , 299-319, (1994) Clofarabine was the latest new drug to be approved, in 2004, for relapsed or refractory acute lymphoblastic leukaemia (ALL). To investigate its value in the frontline treatment of ALL we applied clofa... |
| (3E,8R,9S,10R,13S,14S,17R)-13-Ethyl-17-ethynyl-3-(hydroxyimino)-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl acetate |
| (+)-13-Ethyl-17-hydroxy-18,19-dinor-17a-pregn-4-en-20-yn-3-one Oxime Acetate (Ester) |
| d-17beta-acetoxy-13beta-ethyl-17alpha-ethinyl-gon-4en-3-one oxime |
| dexnorgestrelacetime |
| Norgestimat |
| Norgestimate |
| NORGESTIMATE,USP STANDARD |
| levonorgestrel acetate 3-oxime |
| (3E,8R,9S,10R,13S,14S,17R)-17-Ethinyl-13-ethyl-3-(hydroxyimino)-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-ylacetat |
| acétate de (3E,8R,9S,10R,13S,14S,17R)-13-éthyl-17-éthynyl-3-(hydroxyimino)-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tétradécahydro-1H-cyclopenta[a]phénanthrén-17-yle |
![(8R,9S,10R,13S,14S,17R)-13-Ethyl-17-ethynyl-3-oxo-2,3,6,7,8,9,10, 11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-1 7-yl acetate (non-preferred name) structure](https://image.chemsrc.com/caspic/072/13732-69-9.png)

