Cryptotanshinone

Cryptotanshinone structure
|
Common Name | Cryptotanshinone | ||
|---|---|---|---|---|
| CAS Number | 35825-57-1 | Molecular Weight | 296.360 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 459.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H20O3 | Melting Point | 184-185ºC | |
| MSDS | Chinese USA | Flash Point | 203.4±28.8 °C | |
| Symbol |


GHS06, GHS09 |
Signal Word | Danger | |
Use of CryptotanshinoneCryptotanshinone is a natural compound extracted from the root of Salvia miltiorrhiza Bunge that shows antitumor activities. Cryptotanshinone inhibits STAT3 with an IC50 of 4.6 μM. |
| Name | Cryptotanshinone |
|---|---|
| Synonym | More Synonyms |
| Description | Cryptotanshinone is a natural compound extracted from the root of Salvia miltiorrhiza Bunge that shows antitumor activities. Cryptotanshinone inhibits STAT3 with an IC50 of 4.6 μM. |
|---|---|
| Related Catalog | |
| Target |
STAT3:4.6 μM (IC50) |
| In Vitro | Cryptotanshinone significantly inhibits STAT3-dependent luciferase activity, the STAT3 Tyr705 phosphorylation and the dimerization of STAT3, compared to tanshinone IIA which exhibits no activity. Cryptotanshinone (7 μM) dramatically blocks STAT3 Tyr705 phosphorylation but not STAT3 Ser727 phosphorylation in DU145 cells, and significantly inhibits JAK2 phosphorylation with IC50 of appr 5 μM without affecting the phosphorylation of upstream kinases c-Src and EGFR, suggesting the inhibition of STAT3 Tyr705 phosphorylation might due to a direct mechanism probably by binding to the SH2 domain of STAT3. Cryptotanshinone significantly inhibits the proliferation of DU145 prostate cancer cells harboring constitutively active STAT3 with GI50 of 7 μM by blocking STAT3 activity, which leads to the down-regulation of cyclin D1, Bcl-xL, and survivin, subsequently the accumulation in the G0-G1 phase. Cryptotanshinone exhibits less growth inhibitory effect on PC3, LNCaP and MDA-MB-468 cells[1]. Cryptotanshinone significantly attenuates the in vitro hormonal effects of DEX on ovaries, as indicated by a significant decrease in T and an increase in P levels in the culture medium. Cryptotanshinone significantly increases the levels of phosphorylated AKT2 and GSK3β in the DEX-treated ovaries[2]. Cotreatment with imatinib and Cryptotanshinone shows a significant synergistic killing effect in both imatinib sensitive and resistant CML cell lines, as well as primary CML cells[3]. |
| In Vivo | Cryptotanshinone reverses the ovarian IR and significantly increases 2-deoxy-D-[1,2-3H]-glucose uptake in all examined tissues from the DEX-treated mice. Cryptotanshinone significantly reduces the ovulation rate and plasma E2 and P levels[2]. Cryptotanshinone administration significantly reduces the body weight and food intake of ob/ob mice (C57BL/6J-Lepob) and diet-induced obese (DIO) mice in a dose-dependent manner. Cryptotanshinone causes noticeably less fat in the adipose tissues, significant reductions of serum triglycerides and cholesterol levels, and 2.5- to 3-fold higher AMPK activity of the skeletal muscles than in the control mice. Oral administration of Cryptotanshinone at 600 mg/kg/day produces dramatic reductions in blood glucose levels of ob/ob mice (C57BL/6J-Lepob), db/db mice (C57BL/KsJ-Leprdb), and ZDF rats, which occur after 3 days and persist over the entirety of the monitoring period[4]. |
| Kinase Assay | HCT-116 cells are transiently transfected with reporter plasmid having the STAT3-binding element for regulating luciferase assay. Cells are treated with Cryptotanshinone for 24 hours at a concentration range of 0.2 to 50 μM. After treatment, cells are harvested in 20 μL of passive lysis buffer and luciferase activity is evaluated by the Dual Luciferase Reporter Assay kit on Wallac Victor2. The concentration of Cryptotanshinone that inhibits the luciferase activity by 50% represents IC50 value. |
| Cell Assay | The MTT assay is used for the assessment of cell growth inhibition as described previously. Cells are seeded at a density of 8000 cells per well in 96-well plates in RPMI-1640 containing 10% FBS. Different concentrations of imatinib and CPT are added and incubated for another 24 h. Then, 20 μL of MTT are added into each well and the absorbance at 570 nm is measured on an enzyme linked immunosorbent assay (ELISA) plate reader. The coefficient of drug interaction (CDI) between imatinib and CPT is determined according to a previous study. The calculated method is as follows: CDI/AB/(A × B). According to the absorbance of each group, AB is the ratio of the combination group to the control group; A or B is the ratio of the single agent group to the control group. CDI < 1 indicates a synergistic effect; CDI=1 indicates an additive effect; CDI > 1 indicates an antagonistic effect. In the combination treatment group, the concentrations of CPT are arbitrarily designated according to the 50% inhibitory concentration (IC50) value. Then, the cell viabilities are determined after treatment with different concentrations of imatinib plus CPT (constant CPT concentration for one cell type). Finally, the combination IC50 values of imatinib are calculated, and are represented in Table I. The primary CML cells CP1 to CP3 are isolated from patients in chronic phase, while BC1 and BC2 are isolated from patients in blast crisis. Three independent sets of experiments are performed. The IC50 values are presented as the mean±standard deviation (SD). |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 459.0±45.0 °C at 760 mmHg |
| Melting Point | 184-185ºC |
| Molecular Formula | C19H20O3 |
| Molecular Weight | 296.360 |
| Flash Point | 203.4±28.8 °C |
| Exact Mass | 296.141235 |
| PSA | 43.37000 |
| LogP | 4.93 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.603 |
| Storage condition | Store at RT |
| Symbol |


GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H410 |
| Precautionary Statements | P273-P301 + P310-P501 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | T,N |
| Risk Phrases | 25-50/53 |
| Safety Phrases | 45-60-61 |
| RIDADR | UN2811 - class 6.1 - PG 3 - EHS - Toxic solids, organic, n.o.s., HI: all |
| RTECS | SF8282645 |
| HS Code | 2932999099 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Enhanced miR-210 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3.
Hum. Reprod. 30(3) , 632-41, (2015) What are the roles of the microRNA miR-210-an miRNA that is up-regulated in endometriotic cyst stromal cells (ECSCs)-in the pathogenesis of endometriosis?Up-regulated miR-210 expression in ECSCs is in... |
|
|
Tanshinone I increases CYP1A2 protein expression and enzyme activity in primary rat hepatocytes.
Phytomedicine 19(2) , 169-76, (2012) This study investigated the effects of Danshen and its active ingredients on the protein expression and enzymatic activity of CYP1A2 in primary rat hepatocytes. The ethanolic extract of Danshen roots ... |
|
|
In vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica.
Bioorg. Med. Chem. 19 , 4876-81, (2011) Phytochemical investigations of the n-hexane extract from the roots of Peltodon longipes (Lamiaceae) resulted in the isolation of 12 known abietane diterpenes (1-12). Structures were established on th... |
| TANSHINONE,CRYPTO |
| (R)-1,2,6,7,8,9-Hexahydro-1,6,6-trimethyl-phenanthro(1,2-b)furan-10,11-dione |
| MFCD00210488 |
| Phenanthro[1,2-b]furan-10,11-dione, 1,2,6,7,8,9-hexahydro-1,6,6-trimethyl-, (1R)- |
| Cryptotanshinone |
| Cryptotanshine |
| (R)-(−)-1,6,6-Trimethyl-1,2,6,7,8,9-hexahydrophenanthro[1,2-b]furan-10,11-dione |
| (1R)-1,6,6-Trimethyl-1,2,6,7,8,9-hexahydrophenanthro[1,2-b]furan-10,11-dione |
| Phenanthro(1,2-b)furan-10,11-dione, 1,2,6,7,8,9-hexahydro-1,6,6-trimethyl-, (R)- |
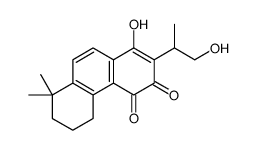 CAS#:109664-02-0
CAS#:109664-02-0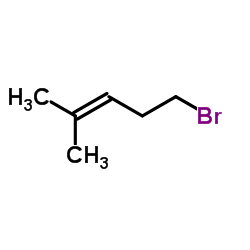 CAS#:2270-59-9
CAS#:2270-59-9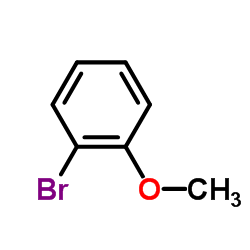 CAS#:578-57-4
CAS#:578-57-4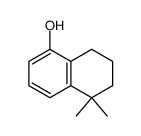 CAS#:171979-69-4
CAS#:171979-69-4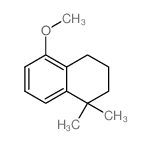 CAS#:33214-70-9
CAS#:33214-70-9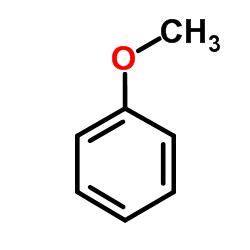 CAS#:100-66-3
CAS#:100-66-3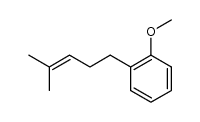 CAS#:171979-70-7
CAS#:171979-70-7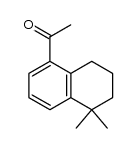 CAS#:171979-68-3
CAS#:171979-68-3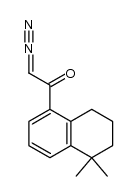 CAS#:171979-67-2
CAS#:171979-67-2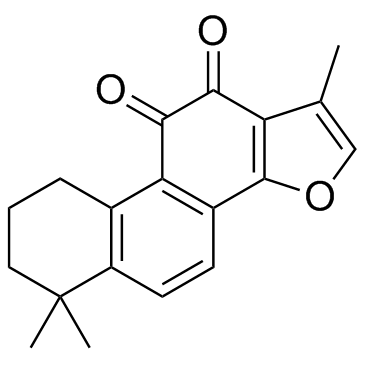 CAS#:568-72-9
CAS#:568-72-9
