10074-G5
10074-G5 structure
|
Common Name | 10074-G5 | ||
|---|---|---|---|---|
| CAS Number | 413611-93-5 | Molecular Weight | 332.313 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 538.6±60.0 °C at 760 mmHg | |
| Molecular Formula | C18H12N4O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 279.5±32.9 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of 10074-G510074-G5 is an inhibitor of c-Myc-Max dimerization with an IC50 of 146 μM. |
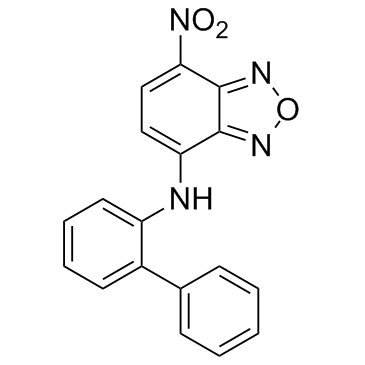
| Name | 4-nitro-N-(2-phenylphenyl)-2,1,3-benzoxadiazol-7-amine |
|---|---|
| Synonym | More Synonyms |
| Description | 10074-G5 is an inhibitor of c-Myc-Max dimerization with an IC50 of 146 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 15.6 μM (Daudi cells), 13.5 μM (HL-60 cells)[1], 146 μM (c-Myc–Max)[2] |
| In Vitro | 10074-G5 inhibits the growth of Daudi Burkitt's lymphoma cells and disruptes c-Myc/Max dimerization. The IC50 values against Daudi and HL-60 cells are 15.6 and 13.5 μM, respectively[1]. 10074-G5 binds the Myc peptide Myc353-437 with a Kd value of 2.8 μM in the region Arg363-Ile381. 10074-G5 binds in a cavity that is created by a kink (Asp379-Ile381) in the N-terminus of an induced helical domain (Leu370–Arg378)[3]. |
| In Vivo | The plasma half-life of 10074-G5 in mice treated with 20 mg/kg i.v. is 37 min, and peak plasma concentration was 58 μM, which is 10-fold higher than peak tumor concentration[1]. |
| Cell Assay | 10074-G5 is dissolved in DMSO and diluted with culture medium. Daudi cells or HL-60 cells in logarithmic growth are treated with 10074-G5 (1-100 μM). After 72 h, 50 μL of 1 mg/mL MTT is added to each well and incubated for 4 h. At the end of the incubation, medium containing drug and MTT is removed from each well, and 100 μl of DMSO is added, followed by shaking for 5 min. The absorbance at 570 nm is read[1]. |
| Animal Admin | Mice: C.B-17 SCID mice bearing Daudi xenografts are stratified into the following groups (10 mice/group): control; vehicle control (0.01 ml/g body weight, once daily for 5 days); positive control, doxorubicin (2.5 mg/kg/dose, one dose every 4 days for three doses); and 10074-G5 (20 mg/kg/dose, once daily for 5 days). Mice are dosed intravenously on the appropriate schedule, and body weights and tumor volumes are recorded twice weekly[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 538.6±60.0 °C at 760 mmHg |
| Molecular Formula | C18H12N4O3 |
| Molecular Weight | 332.313 |
| Flash Point | 279.5±32.9 °C |
| Exact Mass | 332.090942 |
| PSA | 96.77000 |
| LogP | 4.97 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.719 |
| Storage condition | -20℃ |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319-H335 |
| Precautionary Statements | P261-P301 + P310-P305 + P351 + P338 |
| RIDADR | UN 2811 6.1 / PGIII |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Inhibition of c-MYC with involvement of ERK/JNK/MAPK and AKT pathways as a novel mechanism for shikonin and its derivatives in killing leukemia cells.
Oncotarget 6 , 38934-51, (2015) Leukemia remains life-threatening despite remarkable advances in chemotherapy. The poor prognosis and drug resistance are challenging treatment. Novel drugs are urgently needed. Shikonin, a natural na... |
|
|
In vivo quantification and perturbation of Myc-Max interactions and the impact on oncogenic potential.
Oncotarget 5(19) , 8869-78, (2014) The oncogenic bHLH-LZ transcription factor Myc forms binary complexes with its binding partner Max. These and other bHLH-LZ-based protein-protein interactions (PPI) in the Myc-Max network are essentia... |
|
|
Structurally diverse c-Myc inhibitors share a common mechanism of action involving ATP depletion.
Oncotarget 6 , 15857-70, (2015) The c-Myc (Myc) oncoprotein is deregulated in a large proportion of diverse human cancers. Considerable effort has therefore been directed at identifying pharmacologic inhibitors as potential anti-neo... |
| N-[1,1 inverted exclamation marka-Biphenyl-2-yl]-7-nitro-2,1,3-Benzoxadiazol-4-amine |
| N-2-Biphenylyl-7-nitro-2,1,3-benzoxadiazol-4-amine |
| 2,1,3-Benzoxadiazol-4-amine, N-[1,1'-biphenyl]-2-yl-7-nitro- |
| N-(2-Biphenylyl)-7-nitro-2,1,3-benzoxadiazol-4-amine |
| Biphenyl-2-yl-(7-nitro-benzo[1,2,5]oxadiazol-4-yl)-amine |
| 7-nitro-N-(2-phenylphenyl)-2,1,3-benzoxadiazol-4-amine |
| 10074-G5 |