Boldine
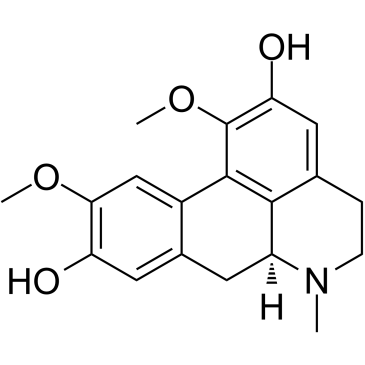
Boldine structure
|
Common Name | Boldine | ||
|---|---|---|---|---|
| CAS Number | 476-70-0 | Molecular Weight | 327.374 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 529.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C19H21NO4 | Melting Point | 162-164ºC | |
| MSDS | Chinese USA | Flash Point | 273.9±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of BoldineBoldine is an aporphine isoquinoline alkaloid extracted from the root of Litsea cubeba and also possesses these properties, including antioxidant, anti-inflammatory and cytoprotective effects. Boldine suppresses osteoclastogenesis, improves bone destruction by down-regulating the OPG/RANKL/RANK signal pathway and may be a potential therapeutic agent for rheumatoid arthritis[1]. |
| Name | (6aS)-1,10-dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-2,9-diol |
|---|---|
| Synonym | More Synonyms |
| Description | Boldine is an aporphine isoquinoline alkaloid extracted from the root of Litsea cubeba and also possesses these properties, including antioxidant, anti-inflammatory and cytoprotective effects. Boldine suppresses osteoclastogenesis, improves bone destruction by down-regulating the OPG/RANKL/RANK signal pathway and may be a potential therapeutic agent for rheumatoid arthritis[1]. |
|---|---|
| Related Catalog | |
| Target |
OPG/RANKL/RANK[1] |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 529.3±50.0 °C at 760 mmHg |
| Melting Point | 162-164ºC |
| Molecular Formula | C19H21NO4 |
| Molecular Weight | 327.374 |
| Flash Point | 273.9±30.1 °C |
| Exact Mass | 327.147064 |
| PSA | 62.16000 |
| LogP | 2.32 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.639 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 1-20-24/25-45-36-26 |
| RIDADR | UN 1544 |
| WGK Germany | 3 |
| RTECS | CE0750000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
~% 
Boldine CAS#:476-70-0 |
| Literature: Nakasato; Nomura Chemical and Pharmaceutical Bulletin, 1959 , vol. 7, p. 780,784 Chem.Abstr., 1960 , p. 4646 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
|
Applied biological and physicochemical activity of isoquinoline alkaloids: oxoisoaporphine and boldine.
Molecules 17(9) , 10958-70, (2012) The aim of this study was to determine the electronic influence of substituent groups and annelated rings such as oxazole-oxazinone on the physicochemical and photoprotection, antioxidant capacity, to... |
|
|
Are extraction methods in quantitative assays of pharmacopoeia monographs exhaustive? A comparison with pressurized liquid extraction.
Planta Med. 72(12) , 1157-62, (2006) The extraction methods in selected monographs of the European and the Swiss Pharmacopoeia were compared to pressurized liquid extraction (PLE) with respect to the yield of constituents to be dosed in ... |
|
|
8-NH2-boldine, an antagonist of alpha1A and alpha1B adrenoceptors without affinity for the alpha1D subtype: structural requirements for aporphines at alpha1-adrenoceptor subtypes.
Planta Med. 71(10) , 897-903, (2005) Structure-activity analysis of 21 aporphine derivatives was performed by examining their affinities for cloned human alpha (1A), alpha (1B) and alpha (1D) adrenoceptors (AR) using membranes prepared f... |
| Boldine chloroform |
| 4H-Dibenzo[de,g]quinoline-2,9-diol, 5,6,6a,7-tetrahydro-1,10-dimethoxy-6-methyl- |
| Boldin |
| 2,6-dihydroxy-3,5-dimethoxyaporphine |
| 5,6,6a,7-Tetrahydro-1,10-dimethoxy-6-methyl-4H-dibenzo[de,g]quinoline-2,9-diol |
| EINECS 207-509-9 |
| 1,10-dimethoxy-2,9-dihydroxyaporphine |
| Uniboldina |
| MFCD00135040 |
| 2,9-Dihydroxy-1,10-dimethoxyaporphine |
| 1,10-Dimethoxy-6aa-aporphine-2,9-diol |
| dl-Boldine |
| Boldine |
| 1,10-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-2,9-diol |


 CAS#:475-81-0
CAS#:475-81-0
