Astragalin
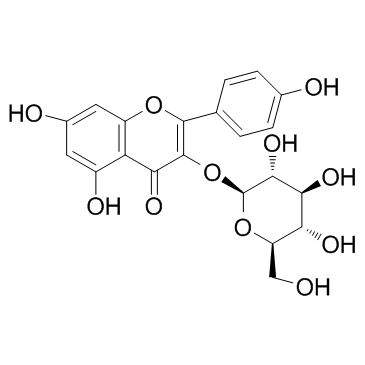
Astragalin structure
|
Common Name | Astragalin | ||
|---|---|---|---|---|
| CAS Number | 480-10-4 | Molecular Weight | 448.377 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 823.2±65.0 °C at 760 mmHg | |
| Molecular Formula | C21H20O11 | Melting Point | 223-229ºC | |
| MSDS | Chinese USA | Flash Point | 291.6±27.8 °C | |
Use of AstragalinAstragalin (kaempferol-3-O-glucoside) is a flavonoid with anti-inflammatory activity and newly found in persimmon leaves and green tea seeds.IC50 value:Target: in vitro: Astragalin nontoxic at ≤ 20 μM suppressed cellular induction of Toll-like receptor 4 (TLR4) and ROS production enhanced by LPS. Both LPS and H2O2 induced epithelial eotaxin-1 expression, which was blocked by astragalin. LPS activated and induced PLCγ1, PKCβ2, and NADPH oxidase subunits of p22phox and p47phox in epithelial cells and such activation and induction were demoted by astragalin or TLR4 inhibition antagonizing eotaxin-1 induction. H2O2-upregulated phosphorylation of JNK and p38 MAPK was dampened by adding astragalin to epithelial cells, while this compound enhanced epithelial activation of Akt and ERK. H2O2 and LPS promoted epithelial apoptosis concomitant with nuclear condensation or caspase-3 activation, which was blunted by astragalin [1]. astragalin suppressed the expression of tumor necrosis factor α, interleukin 6, and nitric oxide in a dose-dependent manner in mMECs [2]. astragalin attenuated the infiltration of inflammatory cells, the activity of myeloperoxidase (MPO) and the expression of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in a dose-dependent manner. Additionally, Western blotting results showed that astragalin efficiently blunt decreased nuclear factor-kappaB (NF-κB) activation by inhibiting the degradation and phosphorylation of IκBα and the nuclear translocation of p65 [3]. Astragalin significantly reduced LPS-induced expression of iNOS, COX-2 and cytokines/chemokines, and production of NO in J774A.1 mouse macrophages. Astragalin inhibited LPSinduced activation of NF-κB as indicated by inhibition of degradation of IκBα, nuclear translocation of NF-κB, and NF-κB dependent gene reporter assay [4].in vivo: Mice were injected intraperitoneally (i.p.) with lipopolysaccharide (LPS) (dose range: 5-40 mg/kg). pretreatment with astragalin can improve survival during lethal endotoxemia and attenuate inflammatory responses in a murine model of lipopolysaccharide-induced acute lung injury [4]. |
| Name | kaempferol 3-O-glucoside |
|---|---|
| Synonym | More Synonyms |
| Description | Astragalin (kaempferol-3-O-glucoside) is a flavonoid with anti-inflammatory activity and newly found in persimmon leaves and green tea seeds.IC50 value:Target: in vitro: Astragalin nontoxic at ≤ 20 μM suppressed cellular induction of Toll-like receptor 4 (TLR4) and ROS production enhanced by LPS. Both LPS and H2O2 induced epithelial eotaxin-1 expression, which was blocked by astragalin. LPS activated and induced PLCγ1, PKCβ2, and NADPH oxidase subunits of p22phox and p47phox in epithelial cells and such activation and induction were demoted by astragalin or TLR4 inhibition antagonizing eotaxin-1 induction. H2O2-upregulated phosphorylation of JNK and p38 MAPK was dampened by adding astragalin to epithelial cells, while this compound enhanced epithelial activation of Akt and ERK. H2O2 and LPS promoted epithelial apoptosis concomitant with nuclear condensation or caspase-3 activation, which was blunted by astragalin [1]. astragalin suppressed the expression of tumor necrosis factor α, interleukin 6, and nitric oxide in a dose-dependent manner in mMECs [2]. astragalin attenuated the infiltration of inflammatory cells, the activity of myeloperoxidase (MPO) and the expression of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in a dose-dependent manner. Additionally, Western blotting results showed that astragalin efficiently blunt decreased nuclear factor-kappaB (NF-κB) activation by inhibiting the degradation and phosphorylation of IκBα and the nuclear translocation of p65 [3]. Astragalin significantly reduced LPS-induced expression of iNOS, COX-2 and cytokines/chemokines, and production of NO in J774A.1 mouse macrophages. Astragalin inhibited LPSinduced activation of NF-κB as indicated by inhibition of degradation of IκBα, nuclear translocation of NF-κB, and NF-κB dependent gene reporter assay [4].in vivo: Mice were injected intraperitoneally (i.p.) with lipopolysaccharide (LPS) (dose range: 5-40 mg/kg). pretreatment with astragalin can improve survival during lethal endotoxemia and attenuate inflammatory responses in a murine model of lipopolysaccharide-induced acute lung injury [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 823.2±65.0 °C at 760 mmHg |
| Melting Point | 223-229ºC |
| Molecular Formula | C21H20O11 |
| Molecular Weight | 448.377 |
| Flash Point | 291.6±27.8 °C |
| Exact Mass | 448.100555 |
| PSA | 190.28000 |
| LogP | 1.95 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.774 |
| Storage condition | ?20°C |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: Kaempferol 3-glucoside REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline.
CAS-No.: 480-10-4 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances SECTION 2: Hazards identification Classification of the substance or mixture Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. This substance is not classified as dangerous according to Directive 67/548/EEC. Label elements The product does not need to be labelled in accordance with EC directives or respective national laws. Other hazards - none SECTION 3: Composition/information on ingredients Substances Formula: C21H20O11 Molecular Weight: 448,38 g/mol CAS-No.: 480-10-4 No components need to be disclosed according to the applicable regulations. SECTION 4: First aid measures Description of first aid measures If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. In case of skin contact Wash off with soap and plenty of water. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Avoid dust formation. Avoid breathing vapours, mist or gas. For personal protection see section 8. Environmental precautions Do not let product enter drains. Methods and materials for containment and cleaning up Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: -20 °C Specific end use(s) Apart from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls General industrial hygiene practice. Personal protective equipment Eye/face protection Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Full contact Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Z677272, Size M) Splash contact Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Z677272, Size M) data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 87300, test method: EN374 If used in solution, or mixed with other substances, and under conditions which differ from EN 374, contact the supplier of the CE approved gloves. This recommendation is advisory only and must be evaluated by an industrial hygienist and safety officer familiar with the specific situation of anticipated use by our customers. It should not be construed as offering an approval for any specific use scenario. Body Protection Choose body protection in relation to its type, to the concentration and amount of dangerous substances, and to the specific work-place., The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Respiratory protection is not required. Where protection from nuisance levels of dusts are desired, use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Do not let product enter drains. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: crystalline Colour: light yellow, white b) Odourodourless c) Odour Thresholdno data available d) pHno data available e) Melting point/freezing312,58 °C point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity no data available Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity no data available Ames test S. typhimurium Result: negative Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: DJ3080000 To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects no data available SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15: Regulatory information This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006. Safety, health and environmental regulations/legislation specific for the substance or mixture no data available Chemical Safety Assessment For this product a chemical safety assessment was not carried out SECTION 16: Other information Further information Copyright 2014 Co. LLC. License granted to make unlimited paper copies for internal use only. The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Corporation and its Affiliates shall not be held liable for any damage resulting from handling or from contact with the above product. See and/or the reverse side of invoice or packing slip for additional terms and conditions of sale. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Precursor 6 | |
|---|---|
| DownStream 8 | |
|
Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performance counter-current chromatography.
J. Chromatogr. A. 1218(36) , 6206-11, (2011) Flaveria bidentis (L.) Kuntze is an annual alien weed of Flaveria Juss. (Asteraceae) in China. Bioactive compounds, mainly flavonol glycosides and flavones from F. bidentis (L.) Kuntze, have been stud... |
|
|
A sensitive LC-MS/MS method for simultaneous determination of six flavonoids in rat plasma: application to a pharmacokinetic study of total flavonoids from mulberry leaves.
J. Pharm. Biomed. Anal. 84 , 189-95, (2013) A simple and sensitive LC-MS/MS method has been developed and validated for the determination of rutin, isoquercitrin, astragalin, quercetin, kaempferol and isorhamnetin in rat plasma using naringin a... |
|
|
[Study on the chemical constituets in ethyl acetante extraction from semen litchi].
Zhong Yao Cai 35(1) , 64-6, (2012) To study the chemical constituents in ethyl acetate extraction of Semen Litchi.The compounds were isolated and purified by column chromatography on silica gel and Sephadex LH-20 coupled with preparati... |
| 5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl b-D-glucopyranoside |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl β-D-glucopyranoside |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl-β-D-glucopyranoside |
| Kaempferol-3-O-D-glucopyranoside |
| 4H-1-Benzopyran-4-one, 3-(β-D-glucopyranosyloxy)-5,7-dihydroxy-2-(4-hydroxyphenyl)- |
| Kaempferol-3-β-glucopyranoside |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one |
| Kaempferol-3-O-glucoside |
| Kaempferol 3-O-β-D-glucoside |
| 5,7-dihydroxy-2-(4-hydroxyphenyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one |
| kaempferol 3-O-glucoside |
| Astragaloside |
| MFCD00075932 |
| Kaempferol-3-O-β-D-glucopyranoside |
| Kaempferol-3-β-monoglucoside |
| Kaempferol-3-D-glucoside |
| Kaempferol 3-O-β-D-glucopyranoside |
| 3-Glucosylkaempferol |
| Astragalin |
| 5,7-Dihydroxy-2-(4-hydroxyphényl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]oxy}-4H-chromén-4-one |
| Kaempferol-3-glucoside |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-on |
| Kaempferol 3-glucoside |
 CAS#:133-89-1
CAS#:133-89-1 CAS#:520-18-3
CAS#:520-18-3 CAS#:152390-63-1
CAS#:152390-63-1 CAS#:56317-05-6
CAS#:56317-05-6 CAS#:23405-70-1
CAS#:23405-70-1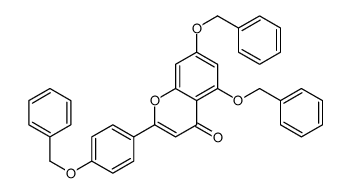 CAS#:96333-59-4
CAS#:96333-59-4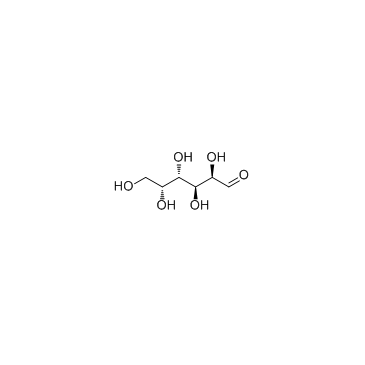 CAS#:59-23-4
CAS#:59-23-4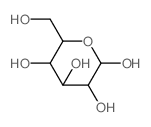 CAS#:10257-28-0
CAS#:10257-28-0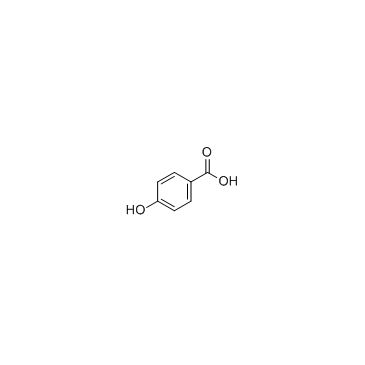 CAS#:99-96-7
CAS#:99-96-7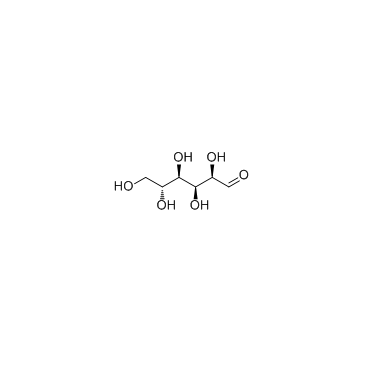 CAS#:50-99-7
CAS#:50-99-7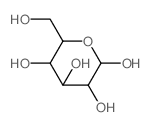 CAS#:2280-44-6
CAS#:2280-44-6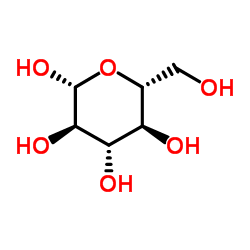 CAS#:492-61-5
CAS#:492-61-5 CAS#:108-73-6
CAS#:108-73-6
