Isoquercitrin
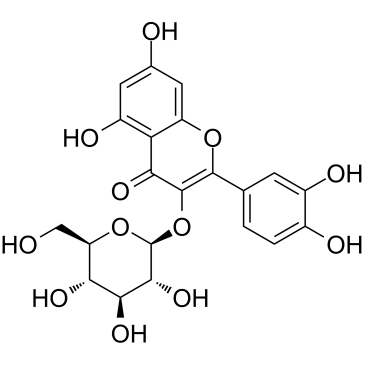
Isoquercitrin structure
|
Common Name | Isoquercitrin | ||
|---|---|---|---|---|
| CAS Number | 482-35-9 | Molecular Weight | 464.376 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 872.6±65.0 °C at 760 mmHg | |
| Molecular Formula | C21H20O12 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 307.5±27.8 °C | |
Use of IsoquercitrinQuercetin-3-glucoside is a naturally occurring polyphenol that has antioxidant, anti-proliferative, and anti-inflammatory properties.Quercetin-3-glucoside alleviates ethanol-induced hepatotoxicity, oxidative stress, and inflammatory responses via the Nrf2/ARE antioxidant signaling pathway[1].Quercetin-3-glucoside regulates the expression of nitric oxide synthase 2 (NO2) via modulating the nuclear factor-κB (NF-κB) transcription regulation system. Quercetin-3-glucoside has high bioavailability and low toxicity, is a promising candidate agent to prevent birth defects in diabetic pregnancies[2]. |
| Name | 3,3′,4′,5,7-Pentahydroxyflavone 3-β-glucoside, Isoquercitrin, Quercetin 3-β-D-glucoside |
|---|---|
| Synonym | More Synonyms |
| Description | Quercetin-3-glucoside is a naturally occurring polyphenol that has antioxidant, anti-proliferative, and anti-inflammatory properties.Quercetin-3-glucoside alleviates ethanol-induced hepatotoxicity, oxidative stress, and inflammatory responses via the Nrf2/ARE antioxidant signaling pathway[1].Quercetin-3-glucoside regulates the expression of nitric oxide synthase 2 (NO2) via modulating the nuclear factor-κB (NF-κB) transcription regulation system. Quercetin-3-glucoside has high bioavailability and low toxicity, is a promising candidate agent to prevent birth defects in diabetic pregnancies[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Quercetin-3-glucoside (5-20 μM; 24 hours) substantially reduces ethanol-induced cytotoxicity , protects hepatic cells against ethanol‐stimulated liver injury[1].Quercetin-3-glucoside (10 μM; pre-treat 1 hour) dramatically downregulates the levels of ethanol-induced iNOS protein expression in HepG2 cells[1]. Cell Viability Assay[1] Cell Line: HepG2 cells Concentration: 5 μM, 10 μM, 20 μM Incubation Time: 24 hours Result: Caused significantly enhanced cell viability as positive controls. Western Blot Analysis[1] Cell Line: HepG2 cells Concentration: 10 μM Incubation Time: 1 hour Result: Decreased ethanol‐ induced iNOS protein expression. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 872.6±65.0 °C at 760 mmHg |
| Molecular Formula | C21H20O12 |
| Molecular Weight | 464.376 |
| Flash Point | 307.5±27.8 °C |
| Exact Mass | 464.095490 |
| PSA | 210.51000 |
| LogP | 1.75 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.803 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | LK8960000 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
|
A nicotinic receptor-mediated anti-inflammatory effect of the flavonoid rhamnetin in BV2 microglia.
Fitoterapia 98 , 11-21, (2014) The alpha7 nicotinic acetylcholine receptor (nAChR) is a potential target in neuroinflammation. Screening a plant extract library identified Solidago nemoralis as containing methyl-quercetin derivativ... |
|
|
New Polyphenols Identified in Artemisiae abrotani herba Extract.
Molecules 20 , 11063-75, (2015) Artemisia abrotanum L. ("southernwood") belongs to the Artemisia genus and it is used in traditional medicine for the treatment of a variety of illnesses. Scarce data is available on the chemical comp... |
|
|
Comparative Studies on Polyphenolic Composition, Antioxidant and Diuretic Effects of Nigella sativa L. (Black Cumin) and Nigella damascena L. (Lady-in-a-Mist) Seeds.
Molecules 20 , 9560-74, (2015) This study was performed to evaluate the phenolic profile, antioxidant and diuretic effects of black cumin and lady-in-a-mist seeds. In the phenolic profile, differences between the two species are si... |
| Quercetin 3-O-β-glucoside |
| trifoliin |
| quercetin 3-O-β-D-glucopyranoside |
| Quercetin 3β-O-glucoside |
| Quercetin 3-β-glucoside |
| 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl β-D-glucopyranoside |
| 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one |
| Quercetin 3-β-D-glucoside |
| 3-O-β-D-Glucopyranosylquercetin |
| Quercetin glucoside |
| Quercetin-3-O-β-D-glucoside |
| Quercetin-3-O-β-glucopyranoside |
| Isoquercetrin |
| Quercetin 3-O-glucoside |
| Quercetin 3-D-glucoside |
| Isoquercitrin |
| isoquercetin |
| quercetin 3-O-β-D-glucoside |
| Hirsutrin |
| isotrifoliin |
| 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3-(β-D-glucopyranosyloxy)-5,7-dihydroxy- |
| Quercetin 3-glucoside |
| Quercetin-3-β-glucopyranoside |
| Quercetin-3-O-glucoside |
| Quercetin 3-β-O-glucoside |
| Quercetin 3-O-glucopyranoside |
 CAS#:153-18-4
CAS#:153-18-4 CAS#:133-89-1
CAS#:133-89-1 CAS#:117-39-5
CAS#:117-39-5 CAS#:6743-87-9
CAS#:6743-87-9![2-(2,2-diphenylbenzo[d][1,3]dioxol-5-yl)-3,5,7-trihydroxy-4H-chroMen-4-one Structure](https://image.chemsrc.com/caspic/359/357194-03-7.png) CAS#:357194-03-7
CAS#:357194-03-7 CAS#:491-70-3
CAS#:491-70-3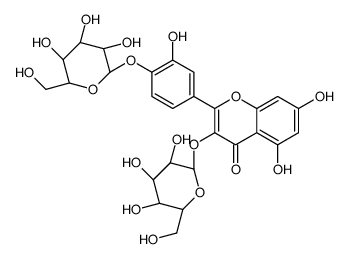 CAS#:29125-80-2
CAS#:29125-80-2 CAS#:1139-97-5
CAS#:1139-97-5 CAS#:855-97-0
CAS#:855-97-0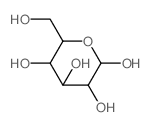 CAS#:2280-44-6
CAS#:2280-44-6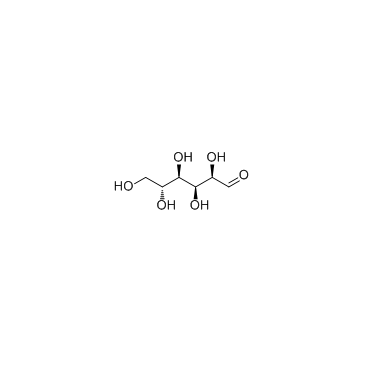 CAS#:50-99-7
CAS#:50-99-7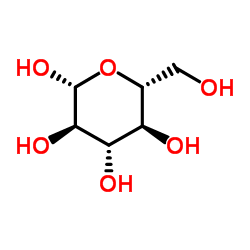 CAS#:492-61-5
CAS#:492-61-5 CAS#:54542-51-7
CAS#:54542-51-7 CAS#:743434-65-3
CAS#:743434-65-3
