Ibudilast
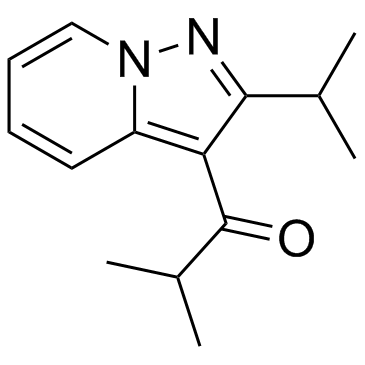
Ibudilast structure
|
Common Name | Ibudilast | ||
|---|---|---|---|---|
| CAS Number | 50847-11-5 | Molecular Weight | 230.305 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C14H18N2O | Melting Point | 53-54°C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of IbudilastIbudilast(KC-404;AV-411;MN-166) is a relatively nonselective phosphodiesterase inhibitor which has been marketed for treating asthma.Target: PDEIbudilast (3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine) is a nonselective inhibitor of cyclic nucleotide phosphodiesterase (PDE). The inhibition of platelet aggregation and vasodilatation by ibudilast may be due to synergistic elevation of intracellular cyclic nucleotides and release of nitric oxide (NO) or prostacyclin from endothelium, rather than direct inhibition of PDE5 or PDE3. Another important property of ibudilast is its antiinflammatory activity possibly associated with potent inhibition of PDE4. Combined with its relaxing effects on bronchial smooth muscle, antiinflammatory actvity of ibudilast could favorably influence pathophysiology of asthma by antagonizing chemical mediators triggering asthmatic attacks [1, 2]. Ibudilast (AV-411) is a non-selective phosphodiesterase inhibitor that is also known to suppress glial cell activation. Preclinical data indicate that ibudilast crosses the blood-brain barrier, is well tolerated, is active on oral administration, reduces glial activation and attenuates pain symptoms in diverse rat models of neuropathic pain. In addition, it enhances acute morphine analgesia and attenuates morphine tolerance and withdrawal. Thus ibudilast may improve opioid efficacy and is a promising therapeutic candidate for neuropathic pain, with a novel mechanism of action [3]. |
| Name | 2-methyl-1-(2-propan-2-ylpyrazolo[1,5-a]pyridin-3-yl)propan-1-one |
|---|---|
| Synonym | More Synonyms |
| Description | Ibudilast(KC-404;AV-411;MN-166) is a relatively nonselective phosphodiesterase inhibitor which has been marketed for treating asthma.Target: PDEIbudilast (3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine) is a nonselective inhibitor of cyclic nucleotide phosphodiesterase (PDE). The inhibition of platelet aggregation and vasodilatation by ibudilast may be due to synergistic elevation of intracellular cyclic nucleotides and release of nitric oxide (NO) or prostacyclin from endothelium, rather than direct inhibition of PDE5 or PDE3. Another important property of ibudilast is its antiinflammatory activity possibly associated with potent inhibition of PDE4. Combined with its relaxing effects on bronchial smooth muscle, antiinflammatory actvity of ibudilast could favorably influence pathophysiology of asthma by antagonizing chemical mediators triggering asthmatic attacks [1, 2]. Ibudilast (AV-411) is a non-selective phosphodiesterase inhibitor that is also known to suppress glial cell activation. Preclinical data indicate that ibudilast crosses the blood-brain barrier, is well tolerated, is active on oral administration, reduces glial activation and attenuates pain symptoms in diverse rat models of neuropathic pain. In addition, it enhances acute morphine analgesia and attenuates morphine tolerance and withdrawal. Thus ibudilast may improve opioid efficacy and is a promising therapeutic candidate for neuropathic pain, with a novel mechanism of action [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Melting Point | 53-54°C |
| Molecular Formula | C14H18N2O |
| Molecular Weight | 230.305 |
| Exact Mass | 230.141907 |
| PSA | 34.37000 |
| LogP | 3.34 |
| Index of Refraction | 1.571 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 28 mg/mL, soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UR0711200 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice.
Eur. J. Pharmacol. 679(1-3) , 75-80, (2012) Over 800,000 Americans abuse the psychomotor stimulant, methamphetamine, yet its abuse is without an approved medication. Methamphetamine induces hypermotor activity, and sensitization to this effect ... |
|
|
Phosphodiesterase inhibitors. Part 1: Synthesis and structure–activity relationships of pyrazolopyridine–pyridazinone PDE inhibitors developed from ibudilast
Bioorg. Med. Chem. Lett. 21(11) , 3307-12, (2011) Ibudilast [1-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-2-methylpropan-1-one] is a nonselective phosphodiesterase inhibitor used clinically to treat asthma. Efforts to selectively develop the PDE3- and ... |
|
|
Ibudilast, a pharmacologic phosphodiesterase inhibitor, prevents human immunodeficiency virus-1 Tat-mediated activation of microglial cells.
PLoS ONE 6(4) , e18633, (2011) Human Immunodeficiency Virus-1 (HIV-1)-associated neurocognitive disorders (HAND) occur, in part, due to the inflammatory response to viral proteins, such as the HIV-1 transactivator of transcription ... |
| KC-404 |
| 1-(2-Isopropylpyrazolo[1,5-a]pyridin-3-yl)-2-methyl-1-propanone |
| MFCD00864808 |
| Ketas (TN) |
| 1-Propanone, 2-methyl-1-[2-(1-methylethyl)pyrazolo[1,5-a]pyridin-3-yl]- |
| 2-isopropyl-3-isobutyrylpyrazolo[1,5-a]pyridine |
| Ibudilast (JAN) |
| Ibudilast [INN:JAN] |
| 1-(2-Isopropylpyrazolo[1,5-a]pyridin-3-yl)-2-methylpropan-1-one |
| Ibudilastum |
| Ibudilast |
| Eyevinal |
| Ke Tas |
| Ketas |
| 3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine |
| Ibudilastum [Latin] |
 CAS#:7583-90-6
CAS#:7583-90-6 CAS#:97-72-3
CAS#:97-72-3![2-Isopropyl-pyrazolo[1,5-a]pyridine-3-carboxylic acid structure](https://image.chemsrc.com/caspic/257/126959-38-4.png) CAS#:126959-38-4
CAS#:126959-38-4![2-(2-hydroxy-1-methylethyl)-3-isobutyrylpyrazolo[1,5-a]pyridine structure](https://image.chemsrc.com/caspic/283/101162-39-4.png) CAS#:101162-39-4
CAS#:101162-39-4 CAS#:79-31-2
CAS#:79-31-2![1-(2-Isopropylpyrazolo[1,5-a]pyridin-3-yl)-2-methyl-1-propanol structure](https://image.chemsrc.com/caspic/405/94144-52-2.png) CAS#:94144-52-2
CAS#:94144-52-2![3-(1-chloro-2-methyl-1-propenyl)-2-isopropylpyrazolo[1,5-a]pyridine structure](https://image.chemsrc.com/caspic/491/115619-23-3.png) CAS#:115619-23-3
CAS#:115619-23-3![2-Isopropylpyrazolo[1,5-a]pyridine-3-carbaldehyde structure](https://image.chemsrc.com/caspic/064/59942-92-6.png) CAS#:59942-92-6
CAS#:59942-92-6
