7,4'-Di-O-methylapigenin
Modify Date: 2024-01-04 22:08:53
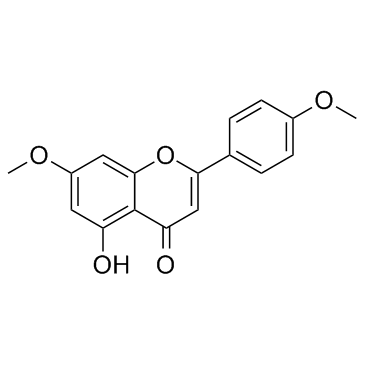
7,4'-Di-O-methylapigenin structure
|
Common Name | 7,4'-Di-O-methylapigenin | ||
|---|---|---|---|---|
| CAS Number | 5128-44-9 | Molecular Weight | 298.290 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 515.2±50.0 °C at 760 mmHg | |
| Molecular Formula | C17H14O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 193.3±23.6 °C | |
Use of 7,4'-Di-O-methylapigeninThe compound 7,4'-Di-O-methylapigenin may be partly responsible for the reported antifungal activity of C. zeyheri, and may serve as a potential source of lead compounds that can be developed as antifungal phytomedicines.And it also showed inhibition of the drug efflux pumps (with IC50 = 51.64 μg/ml).IC50:51.64 μg/ml(Candida albicans drug efflux pumps)[2]In vitro: The isolated 7,4'-Di-O-methylapigenin was further investigated for its inhibitory activity on ABC drug efflux pumps in C. albicans by monitoring an increase in ciprofloxacin, assessing the level of its accumulation, in response to reserpine. There was a higher accumulation of ciprofloxacin in Candida cells in the presence of 7,4'-Di-O-methylapigenin than with reserpine. The compound 7,4'-Di-O-methylapigenine demonstrated the activity in a dose-dependent manner with IC50 value of 51.64 μg/ml. These results support those obtained from synergism assays where by the underlying synergistic antifungal mechanisms could be due to blockage of ABC efflux pumps and increasing the susceptibility of Candida to miconazole.[2]In vivo: In searching for natural products as potential anti-inflammatory agents, 7,4'-Di-O-methylapigenin wasn't evaluated in vivo for its ability to inhibit acute inflammation.[1] |
| Name | apigenin 7,4'-dimethyl ether |
|---|---|
| Synonym | More Synonyms |
| Description | The compound 7,4'-Di-O-methylapigenin may be partly responsible for the reported antifungal activity of C. zeyheri, and may serve as a potential source of lead compounds that can be developed as antifungal phytomedicines.And it also showed inhibition of the drug efflux pumps (with IC50 = 51.64 μg/ml).IC50:51.64 μg/ml(Candida albicans drug efflux pumps)[2]In vitro: The isolated 7,4'-Di-O-methylapigenin was further investigated for its inhibitory activity on ABC drug efflux pumps in C. albicans by monitoring an increase in ciprofloxacin, assessing the level of its accumulation, in response to reserpine. There was a higher accumulation of ciprofloxacin in Candida cells in the presence of 7,4'-Di-O-methylapigenin than with reserpine. The compound 7,4'-Di-O-methylapigenine demonstrated the activity in a dose-dependent manner with IC50 value of 51.64 μg/ml. These results support those obtained from synergism assays where by the underlying synergistic antifungal mechanisms could be due to blockage of ABC efflux pumps and increasing the susceptibility of Candida to miconazole.[2]In vivo: In searching for natural products as potential anti-inflammatory agents, 7,4'-Di-O-methylapigenin wasn't evaluated in vivo for its ability to inhibit acute inflammation.[1] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 515.2±50.0 °C at 760 mmHg |
| Molecular Formula | C17H14O5 |
| Molecular Weight | 298.290 |
| Flash Point | 193.3±23.6 °C |
| Exact Mass | 298.084137 |
| PSA | 68.90000 |
| LogP | 3.40 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.622 |
| Storage condition | -20℃ |
| HS Code | 2914509090 |
|---|
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
| Genkwanin 4'-methyl ether |
| Flavone, 5-hydroxy-4',7-dimethoxy- |
| 4',7-Dimethylapigenin |
| Apigenin dimethyl ether |
| 7,4'-dimethylapigenin |
| 5-Hydroxy-4',7-dimethoxyflavone |
| 5-Hydroxy-4',7-dimethoxy-flavone |
| 5-Hydroxy-7-methoxy-2-(4-methoxyphenyl)-4H-chromen-4-one |
| 7,4'-di-O-methylapigenin |
| 5-hydroxy-7-methoxy-2-(4-methoxyphenyl)chromen-4-one |
| Apigenin 7,4'-dimethyl ether |
| 4H-1-Benzopyran-4-one, 5-hydroxy-7-methoxy-2- (4-methoxyphenyl)- |
| Apigenin 4',7-dimethyl ether |
| 4H-1-Benzopyran-4-one, 5-hydroxy-7-methoxy-2-(4-methoxyphenyl)- |
 CAS#:480-41-1
CAS#:480-41-1 CAS#:74-88-4
CAS#:74-88-4 CAS#:5631-70-9
CAS#:5631-70-9 CAS#:480-44-4
CAS#:480-44-4 CAS#:186581-53-3
CAS#:186581-53-3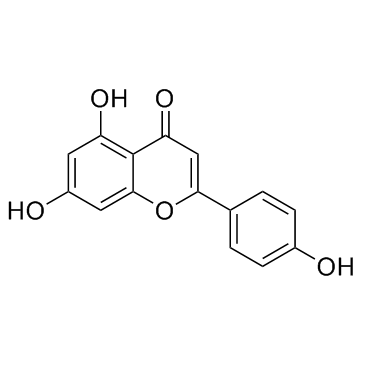 CAS#:520-36-5
CAS#:520-36-5 CAS#:77-78-1
CAS#:77-78-1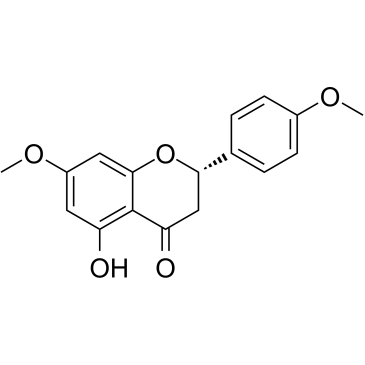 CAS#:29424-96-2
CAS#:29424-96-2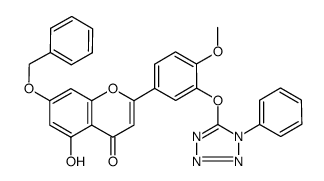 CAS#:771481-08-4
CAS#:771481-08-4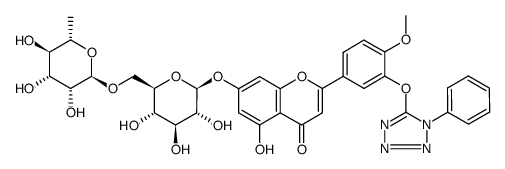 CAS#:771480-96-7
CAS#:771480-96-7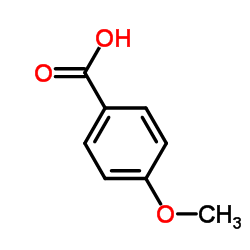 CAS#:100-09-4
CAS#:100-09-4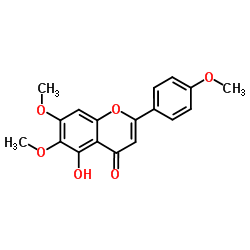 CAS#:19103-54-9
CAS#:19103-54-9 CAS#:520-18-3
CAS#:520-18-3 CAS#:529-53-3
CAS#:529-53-3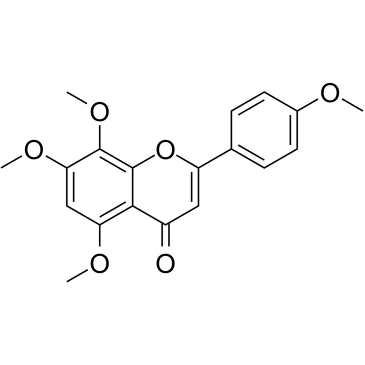 CAS#:6601-66-7
CAS#:6601-66-7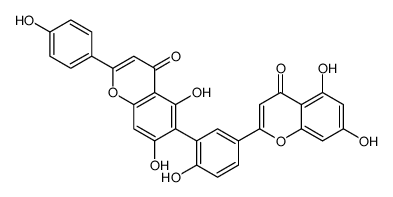 CAS#:49620-13-5
CAS#:49620-13-5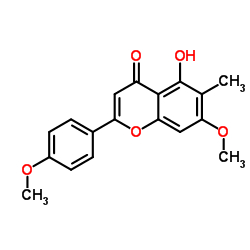 CAS#:5689-38-3
CAS#:5689-38-3
