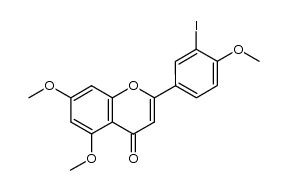
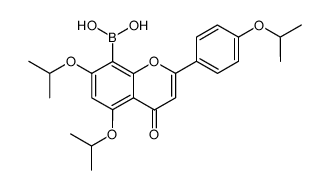
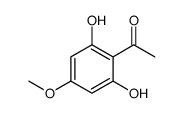
481-46-9
| Name | ginkgetin |
|---|---|
| Synonyms |
7,4'-di-O-methylamentoflavone
Amentoflavone 7,4'-dimethyl ether 4H-1-Benzopyran-4-one, 5,7-dihydroxy-8-[5-(5-hydroxy-7-methoxy-4-oxo-4H-1-benzopyran-2-yl)-2-methoxyphenyl]-2-(4-hydroxyphenyl)- 5,7-Dihydroxy-8-[5-(5-hydroxy-7-methoxy-4-oxo-4H-chromen-2-yl)-2-methoxyphenyl]-2-(4-hydroxyphenyl)-4H-chromen-4-one 4H-1-Benzopyran-4-one, 5,7-dihydroxy-8-(5-(5-hydroxy-7-methoxy-4-oxo-4H-1-benzopyran-2-yl)-2-methoxyphenyl)-2-(4-hydroxyphenyl)- ginkgetin 5,7-dihydroxy-8-[5-(5-hydroxy-7-methoxy-4-oxochromen-2-yl)-2-methoxyphenyl]-2-(4-hydroxyphenyl)chromen-4-one |
| Description | Ginkgetin is a natural biflavonoid isolated from leaves of Ginkgo biloba L; effects of anti-inflammation and anticancer have been reported.IC50 value:Target:in vitro: Ginkgetin inhibits COX-2 dependent phases of prostaglandin D(2) (PGD(2)) generation in bone marrow-derived mast cells (BMMC) in a concentration-dependent manner with IC(50) values of 0.75 microM. Ginkgetin consistently inhibited the production of leukotriene C(4) (LTC(4)) in a dose dependent manner, with an IC(50) value of 0.33 microM. Ginkgetin also inhibited degranulation reaction in a dose dependent manner, with an IC(50) value of 6.52 microM [1]. Ginkgetin inhibited both inducible and constitutively activated STAT3 and blocked the nuclear translocation of p-STAT3 in DU-145 prostate cancer cells. Furthermore, ginkgetin selectively inhibited the growth of prostate tumor cells stimulated with activated STAT3. Ginkgetin induced STAT3 dephosphorylation at Try705 and inhibited its localization to the nucleus, leading to the inhibition of expression of STAT3 target genes such as cell survival-related genes (cyclin D1 and survivin) and anti-apoptotic proteins (Bcl-2 and Bcl-xL) [2]. Ginkgetin suppressed the viability of PC-3 cells in a concentration-dependent manner and also significantly increased the sub-G1 DNA contents of cell cycle in PC-3 cells. Ginkgetin activated caspase-3 and attenuated the expression of survival genes such as Bcl-2, Bcl-xL, survivin and Cyclin D1 at protein and mRNA levels [3]. Ginkgetin (1 - 10 microM) and the biflavonoid mixture (10 - 50 microg/ml), mainly a 1 : 1 mixture of ginkgetin and isoginkgetin, from G. biloba leaves, inhibited production of prostaglandin E2 from lipopolysaccharide-induced RAW 264.7 cells [4].in vivo: Ginkgetin inhibited tumor growth in xenografted nude mice and down-regulated p-STAT3Tyr705 and survivin in tumor tissues [2]. At total doses of 1,000 microg/site on the dorsal skin (15 mm x 15 mm), ginkgetin inhibited prostaglandin E2 production by 65.6 % along with a marked suppression of COX-2 induction. In addition, ginkgetin and the biflavonoid mixture (100 - 1,000 microg/ear) dose-dependently inhibited skin inflammation of croton oil induced ear edema in mice by topical application [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 863.7±65.0 °C at 760 mmHg |
| Melting Point | 336ºC |
| Molecular Formula | C32H22O10 |
| Molecular Weight | 566.511 |
| Flash Point | 287.2±27.8 °C |
| Exact Mass | 566.121277 |
| PSA | 159.80000 |
| LogP | 4.45 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.714 |
| Storage condition | -20°C |
|
~96% 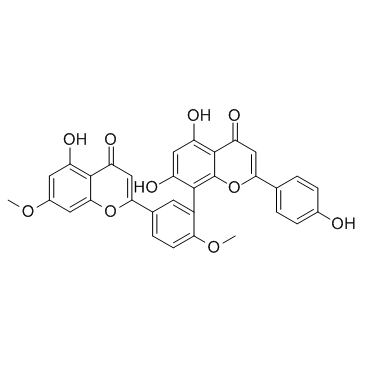
481-46-9 |
| Literature: Muller, Dominique; Fleury, Jean-Pierre Tetrahedron Letters, 1991 , vol. 32, # 20 p. 2229 - 2232 |
|
~% 
481-46-9 |
| Literature: Muller, Dominique; Fleury, Jean-Pierre Tetrahedron Letters, 1991 , vol. 32, # 20 p. 2229 - 2232 |
|
~% 
481-46-9 |
| Literature: Muller, Dominique; Fleury, Jean-Pierre Tetrahedron Letters, 1991 , vol. 32, # 20 p. 2229 - 2232 |