Tetrandrine
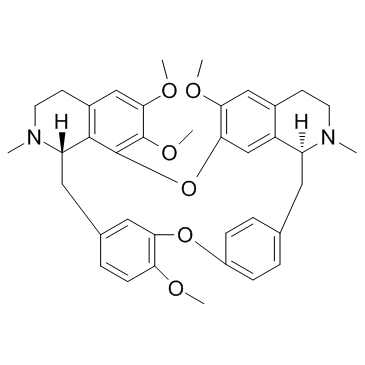
Tetrandrine structure
|
Common Name | Tetrandrine | ||
|---|---|---|---|---|
| CAS Number | 518-34-3 | Molecular Weight | 622.750 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 710.5±60.0 °C at 760 mmHg | |
| Molecular Formula | C38H42N2O6 | Melting Point | 219-222ºC | |
| MSDS | Chinese USA | Flash Point | 175.8±30.1 °C | |
Use of TetrandrineTetrandrine is a bis-benzyl-isoquinoline alkaloid, which inhibits voltage-gated Ca2+ current (ICa) and Ca2+-activated K+ current. |
| Name | D-Tetrandrine |
|---|---|
| Synonym | More Synonyms |
| Description | Tetrandrine is a bis-benzyl-isoquinoline alkaloid, which inhibits voltage-gated Ca2+ current (ICa) and Ca2+-activated K+ current. |
|---|---|
| Related Catalog | |
| Target |
Ca2+ current[1] K+ current[1] |
| In Vitro | The effects of Tetrandrine, a bis-benzyl-isoquinoline alkaloid, on voltage-gated Ca2+ currents (ICa) and on Ca2+-activated K+ current (IK(Ca)) and channels in isolated nerve terminals of the rat neurohypophysis are investigated using patch-clamp techniques. The non-inactivating component of ICa is inhibited by external Tetrandrine in a voltage- and dose-dependent manner, with an IC50=10.1μM. Tetrandrine decreases the channel-open probability, within bursts, with an IC50=0.21 μM[1]. To evaluate the effects of Tetrandrine on HCC cells, Huh7, HCCLM9 and Hep3B cells are treated with 0 (DMSO), 0.5, 1, 2 or 4 μM of Tetrandrine for 24 h. The cell proliferation assay indicates that Tetrandrine exhibits almost no effect on the inhibition of HCC cell proliferation at 0.5-2 μM. However, Tetrandrine inhibits HCC cell migration in a dose-dependent manner. Furthermore, a wound-healing and transwell assay shows that 2 μM Tetrandrine significantly inhibits HCC cell migration and invasion[2]. |
| In Vivo | To evaluate the effect of Tetrandrine on the inhibition of tumor metastasis in vivo, HCCLM9 subcutaneous tumor xenograft models is established with athymic nude mice. When the tumor volume reach approximately 50 mm3, nude mice are orally administered vehicle or Tetrandrine (30 mg/kg) every other day for 37 days. Tetrandrine treatment inhibits tumor growth by reducing the tumor volume and weight[2]. |
| Cell Assay | Huh7, HCCLM9 and Hep3B cells are seeded in a 96-well plate at a cell density of 5 × 103 cells/well. The cells are treated with the indicated concentrations (0-4 μM) of Tetrandrine for 24 h. The cells are subsequently stained with 20 μL of MTS for 1-2 h, and the plates are read at 490 nm on a BioTek ELx800[2]. |
| Animal Admin | Mice[2] Four-week-old male athymic BALB/c nu/nu SPF mice (body weight range from 18 g to 20 g) are used. HCCLM9 WT and HCCLM9 ATG7 KO cells (5 million) resuspended in 0.2 mL of PBS are subcutaneously implanted into the right flank of each mouse. When the tumor volume reach approximately 50 mm3, the tumor-bearing mice are randomly divided into control and treatment groups (n = 6). The control and treatment groups are administered oral injection of vehicle (0.5% methylcellulose) and Tetrandrine at 30 mg/kg of body weight every other day for 37 days. During the treatment, the tumor volumes are measured every day and are calculated. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 710.5±60.0 °C at 760 mmHg |
| Melting Point | 219-222ºC |
| Molecular Formula | C38H42N2O6 |
| Molecular Weight | 622.750 |
| Flash Point | 175.8±30.1 °C |
| Exact Mass | 622.304260 |
| PSA | 61.86000 |
| LogP | 3.55 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.586 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | XE9350000 |
| HS Code | 2942000000 |
|
~98% 
Tetrandrine CAS#:518-34-3 |
| Literature: Kashiwaba, Noriaki; Morooka, Shigeo; Kimura, Michiko; Ono, Minoru; Murakoshi, Yoshie; et al. Heterocycles, 1995 , vol. 41, # 9 p. 2043 - 2048 |
| Precursor 2 | |
|---|---|
| DownStream 9 | |
| HS Code | 2942000000 |
|---|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
[Anti-tumor effect of tanshinone II A, tetrandrine, honokiol, curcumin, oridonin and paeonol on leukemia cell lines].
Sichuan Da Xue Xue Bao. Yi Xue Ban 43(3) , 362-6, (2012) To study the anti-tumor effect of tanshinon II A, tetrandrine, honokiol, curcumin, oridonin and paeonol on leukemia cell lines SUP-B15, K562, CEM, HL-60 and NB4.To study the anti-tumor effect of tansh... |
|
|
Precision-cut liver slices as a model for the early onset of liver fibrosis to test antifibrotic drugs.
Toxicol. Appl. Pharmacol. 274(2) , 328-38, (2014) Induction of fibrosis during prolonged culture of precision-cut liver slices (PCLS) was reported. In this study, the use of rat PCLS was investigated to further characterize the mechanism of early ons... |
| (1S,14S)-9,20,21,25-Tetramethoxy-15,30-dimethyl-7,23-dioxa-15,30-diazaheptacyclo[22.6.2.2.1.1.0.0]hexatriaconta-3,5,8(34),9,11,18(33),19,21,24,26,31,35-dodecaene |
| Tetrandrine |
| (1S,14S)-9,20,21,25-Tetramethoxy-15,30-dimethyl-7,23-dioxa-15,30-diazaheptacyclo[22.6.2.2.1.1.0.0]hexatriaconta-3,5,8(34),9,11,18(33),19,21,24,26,31,35-dodecaen |
| Trandrine |
| Conba |
| MFCD08689909 |
| (1β)-6,6',7,12-Tetramethoxy-2,2'-dimethylberbaman |
| sinomeninea |
| 6,6',7,12-Tetramethoxy-2,2'-dimethylberbaman |
| hanfangchin A |
| TETRANDRIN |
| fanchinine |
| Jinake |
| 6,6',7,12-tetramethoxy-2,2'-dimethyl-berbaman |
| (1S,14S)-9,20,21,25-Tétraméthoxy-15,30-diméthyl-7,23-dioxa-15,30-diazaheptacyclo[22.6.2.2.1.1.0.0]hexatriaconta-3,5,8(34),9,11,18(33),19,21,24,26,31,35-dodécaène |
| (1b)-6,6',7,12-Tetramethoxy-2,2'-dimethylberbaman |
| (S,S)-(+)-Tetrandrine |
| iso-tetrandrine |
| (1β,1'β)-6,6',7,12-Tetramethoxy-2,2'-dimethylberbaman |

 CAS#:83113-25-1
CAS#:83113-25-1 CAS#:83087-72-3
CAS#:83087-72-3 CAS#:436-77-1
CAS#:436-77-1 CAS#:83113-24-0
CAS#:83113-24-0 CAS#:18251-36-0
CAS#:18251-36-0 CAS#:93-51-6
CAS#:93-51-6 CAS#:75-50-3
CAS#:75-50-3 CAS#:3423-07-2
CAS#:3423-07-2
