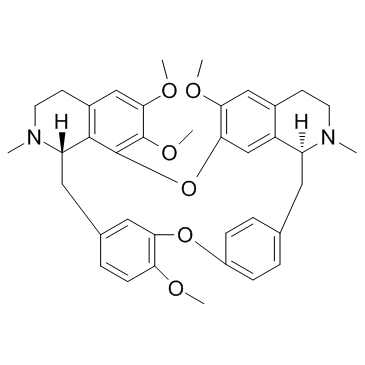
436-77-1
| Name | Fangchinoline |
|---|---|
| Synonyms |
Berbaman-7-ol, 6,6',12-trimethoxy-2,2'-dimethyl-
Fangquinoline FF-0018 d,l-Fangchinolin (S,S)-(+)-tetrandine 12-O-MethylatherosperMoline (1β)-6,6',12-Trimethoxy-2,2'-dimethylberbaman-7-ol Tetrandrine B 6,6',12-Trimethoxy-2,2'-dimethylberbaman-7-ol Hanfangichin B N1799 I06-1344 THALRUGOSINE Fangchinoline 7-o-demethyltetrandrine |
| Description | Fangchinoline is isolated from Stephania tetrandra with extensive biological activities, such as enhancing immunity, anti-inflammatory sterilization and anti-atherosclerosis. Fangchinoline, a novel HIV-1 inhibitor, inhibits HIV-1 replication by impairing gp160 proteolytic processing[1]. Fangchinoline targets Focal adhesion kinase (FAK) and suppresses FAK-mediated signaling pathway in tumor cells which highly expressed FAK[2]. Fangchinoline induces apoptosis and adaptive autophagy in bladder cancer[3]. |
|---|---|
| Related Catalog | |
| Target |
HIV-1 replication[1]; Focal adhesion kinase (FAK)[2]; apoptosis; autophagy[3] |
| In Vitro | Fangchinoline (2.5-40 µM; 24-96 hours) inhibits both T24 and 5637 cells in dose-dependent manner, the IC50 values of Fangchinoline in T24 cells are 19.0 µM (24 h), 12.0 µM (48 h) and 7.57 µM (72 h), and 11.9 µM (24 h), 9.92 µM (48 h) and 7.13 µM (72 h) in 5637 cells[1]. Fangchinoline (5 µM; 24 hours) induces a significant increase in the LC3-II/LC3-I ratio and a decrease in p62 in both T24 and 5637 cells, and causes a significant increase in the cleavage of caspase-3[1]. Cell Viability Assay[3] Cell Line: T24 and 5637 cells Concentration: 2.5 µM; 5 µM; 10 µM; 20 µM; 30 µM; 40 µM Incubation Time: 24 hours; 48 hours; 96 hours Result: Inhibited both T24 and 5637 cells proliferation. Western Blot Analysis[3] Cell Line: T24 and 5637 cells Concentration: 5 µM Incubation Time: 24 hours Result: Incresed LC3-II/LC3-I ratio and the cleavage of caspase-3. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 709.7±60.0 °C at 760 mmHg |
| Molecular Formula | C37H40N2O6 |
| Molecular Weight | 608.723 |
| Flash Point | 383.0±32.9 °C |
| Exact Mass | 608.288635 |
| PSA | 72.86000 |
| LogP | 3.78 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.602 |
| Storage condition | 2-8C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~36%
Detail
|
| Literature: Pachaly; Praest Archiv der Pharmazie, 1982 , vol. 315, # 7 p. 589 - 597 |
|
~36%
Detail
|
| Literature: Pachaly; Praest Archiv der Pharmazie, 1982 , vol. 315, # 7 p. 589 - 597 |
|
~32% 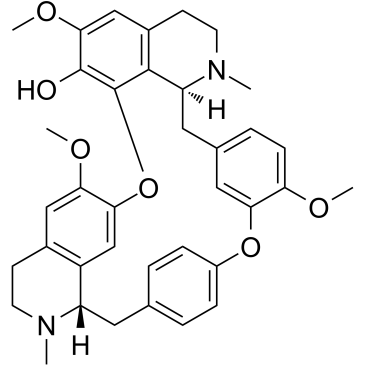
436-77-1 |
| Literature: Kunitomo, Jun-ichi; Oshikata, Megumi; Akasu, Michinori; Ishii, Hisashi Heterocycles, 1992 , vol. 34, # 5 p. 937 - 942 |
|
~35%
Detail
|
| Literature: Pachaly; Praest Archiv der Pharmazie, 1982 , vol. 315, # 7 p. 589 - 597 |
| Precursor 1 | |
|---|---|
| DownStream 2 | |