Hesperidin
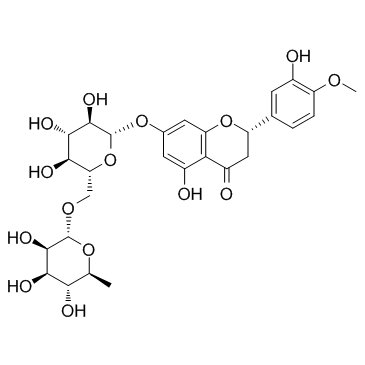
Hesperidin structure
|
Common Name | Hesperidin | ||
|---|---|---|---|---|
| CAS Number | 520-26-3 | Molecular Weight | 610.561 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 930.1±65.0 °C at 760 mmHg | |
| Molecular Formula | C28H34O15 | Melting Point | 250-255 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 305.5±27.8 °C | |
Use of HesperidinHesperidin (HP) is a bioflavonoid that plays a role in plant defense and is abundant in citrus species, such as grapefruit, lemon and orange. Hesperidin is used effectively as a supplemental agent in complementary therapy protocols, since it possesses biological and pharmacological properties as an effective antioxidant, anti-inflammatory, anti-carcinogenic, and anti-hypertensive agent with lipid-lowering activity[1]IC50: hesperidin (IC50=116.68μmo/L))[4]in vitro: hesperidin and linarin are two of the main constituent of Valeriana's extract exhibiting a high affinity to KATP channel, which are related to the control of Ca++ concentration and release of GABA in synaptic nerve terminal, mainly on cells of SN[2]in vivo: Hesperidin was dissolved in 1% carboxymethyl cellulose (CMC) and administered orally at a dose of 50 mg/kg for 10 consecutive days. In the control group, rats were treated with the corn oil and 1% CMC vehicle.[1] |
| Name | hesperidin |
|---|---|
| Synonym | More Synonyms |
| Description | Hesperidin (HP) is a bioflavonoid that plays a role in plant defense and is abundant in citrus species, such as grapefruit, lemon and orange. Hesperidin is used effectively as a supplemental agent in complementary therapy protocols, since it possesses biological and pharmacological properties as an effective antioxidant, anti-inflammatory, anti-carcinogenic, and anti-hypertensive agent with lipid-lowering activity[1]IC50: hesperidin (IC50=116.68μmo/L))[4]in vitro: hesperidin and linarin are two of the main constituent of Valeriana's extract exhibiting a high affinity to KATP channel, which are related to the control of Ca++ concentration and release of GABA in synaptic nerve terminal, mainly on cells of SN[2]in vivo: Hesperidin was dissolved in 1% carboxymethyl cellulose (CMC) and administered orally at a dose of 50 mg/kg for 10 consecutive days. In the control group, rats were treated with the corn oil and 1% CMC vehicle.[1] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 930.1±65.0 °C at 760 mmHg |
| Melting Point | 250-255 °C (dec.)(lit.) |
| Molecular Formula | C28H34O15 |
| Molecular Weight | 610.561 |
| Flash Point | 305.5±27.8 °C |
| Exact Mass | 610.189758 |
| PSA | 234.29000 |
| LogP | 1.78 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.695 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | MK6650000 |
|
~% 
Hesperidin CAS#:520-26-3 |
| Literature: Yamada, Mika; Tanabe, Fujimi; Arai, Norie; Mitsuzumi, Hitoshi; Miwa, Yoshikatsu; Kubota, Michio; Chaen, Hiroto; Kibata, Masayoshi Bioscience, Biotechnology and Biochemistry, 2006 , vol. 70, # 6 p. 1386 - 1394 |
|
~% 
Hesperidin CAS#:520-26-3 |
| Literature: Yamada, Mika; Tanabe, Fujimi; Arai, Norie; Mitsuzumi, Hitoshi; Miwa, Yoshikatsu; Kubota, Michio; Chaen, Hiroto; Kibata, Masayoshi Bioscience, Biotechnology and Biochemistry, 2006 , vol. 70, # 6 p. 1386 - 1394 |
|
~% 
Hesperidin CAS#:520-26-3 |
| Literature: Shimokoriyama Journal of the American Chemical Society, 1957 , vol. 79, p. 4199,4200 |
|
~% 
Hesperidin CAS#:520-26-3 |
| Literature: Belboukhari, Nasser; Cheriti, Abdelkrim; Roussel, Christian; Vanthuyne, Nicolas Natural Product Research, 2010 , vol. 24, # 7 p. 669 - 681 |
| Precursor 4 | |
|---|---|
| DownStream 6 | |
|
Bio-refinery of orange peels waste: a new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin.
Ultrason. Sonochem. 24 , 72-9, (2015) In this study, extraction of essential oil, polyphenols and pectin from orange peel has been optimized using microwave and ultrasound technology without adding any solvent but only "in situ" water whi... |
|
|
Effects of pectinase clarification treatment on phenolic compounds of pummelo (Citrus grandis l. Osbeck) fruit juice.
J. Food Sci. Technol. 52 , 5057-65, (2015) The purpose of this study is to investigate the changes occured on phenolic compounds between two Malaysian varieties of pummelo fruit juice: Ledang (PO55) and Tambun (PO52) post-enzymatic clarificati... |
|
|
Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain.
Food Chem. Toxicol. 74 , 51-9, (2014) D-galactose, a reducing sugar, induces oxidative stress resulting in alteration in mitochondrial dynamics and apoptosis of neurons. Curcumin and hesperidin are antioxidants possessing multimodal funct... |
| usafcf-3 |
| Hesperetin 7-rhamnoglucoside,Hesperitin-7-rutinoside |
| VITAMIN P |
| Hespeidin |
| Hespiridin |
| Hesperiden |
| Neobiletin |
| cirantin |
| MFCD00075663 |
| Hesperetin7-rutinoside |
| Cirontin |
| Hesperidoside |
| Hesperetin7-rhamnoglucoside |
| Hesperidin |
| EINECS 208-288-1 |
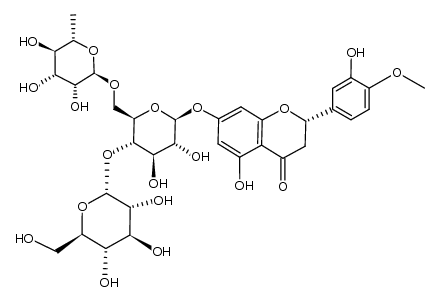


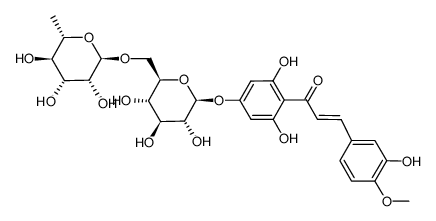
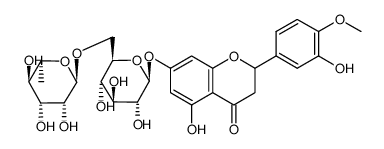
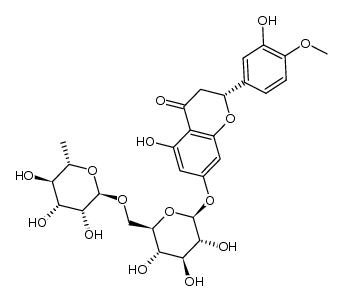
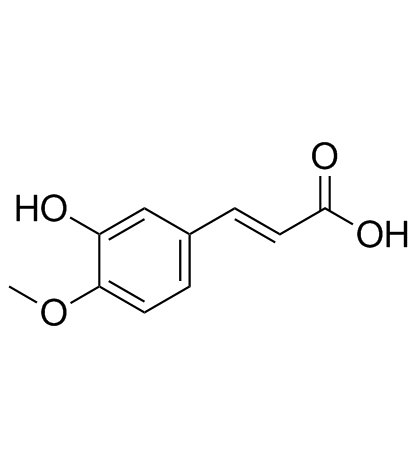 CAS#:537-73-5
CAS#:537-73-5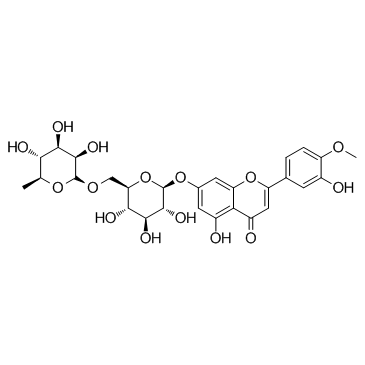 CAS#:520-27-4
CAS#:520-27-4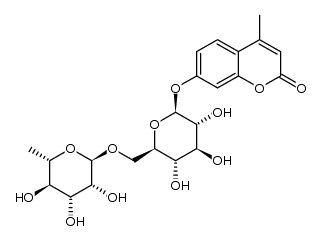 CAS#:1356391-89-3
CAS#:1356391-89-3 CAS#:26184-96-3
CAS#:26184-96-3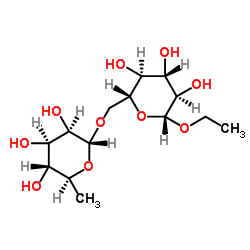 CAS#:187539-57-7
CAS#:187539-57-7
