PENTOSTATIN
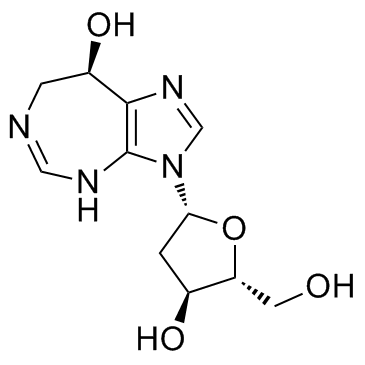
PENTOSTATIN structure
|
Common Name | PENTOSTATIN | ||
|---|---|---|---|---|
| CAS Number | 53910-25-1 | Molecular Weight | 268.269 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 673.1±65.0 °C at 760 mmHg | |
| Molecular Formula | C11H16N4O4 | Melting Point | 220-225ºC | |
| MSDS | Chinese USA | Flash Point | 360.9±34.3 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of PENTOSTATINPentostatin is an irreversible inhibitor of adenosine deaminase with Ki of 2.5 pM. |
| Name | pentostatin |
|---|---|
| Synonym | More Synonyms |
| Description | Pentostatin is an irreversible inhibitor of adenosine deaminase with Ki of 2.5 pM. |
|---|---|
| Related Catalog | |
| Target |
Ki: 2.5 pM (adenosine deaminase) |
| In Vivo | In ECP and Pentostatin (4 mg/m2, i.v.) treatment, all dogs develop granulocytopenia with <500 granulocytes/μL from day 4. Thrombocytopenia (<20,000 platelets/μL) occurs from day 7 after HCT with nadirs of 3000 to 14000 platelets/μL[1]. Pentostatin (2 mg/kg) in combination with cordycepin (2 mg/kg) is 100% effective in the T. evansi-infected mice. There is an increase in levels of some biochemical parameters, especially on liver enzymes, which are accompanied by histological lesions in the liver and kidneys. Pentostatin individually has no curative effect on infected groups[2]. |
| Animal Admin | All recipient dogs are conditioned for transplantation by 920 cGy TBI at 7 cGy/minute using a linear accelerator. Dogs in group A1receive ECP administered on days −2 and −1 with TBI on day 0 and dogs in group A2receive ECP on days −6 and −5, intravenous (IV) infusion of pentostatin at a dose of 4 mg/m2 on days −4 and −3, and TBI on day 0. Donor marrow cells from DLA-nonidentical donors are aspirated under general anesthesia through needles inserted into humeri and femora and stored in heparinized tissue culture medium at 4°C for no more than 6 hours. Within 4 hours of TBI, harvested marrow cells are infused IV into recipients at a median dose of 2.9 (range, 1.9 to 6.1) ×108 total nucleated cells (TNC)/kg. The day of marrow grafting is designated as day 0. In addition to marrow graft, recipients are given IV infusions of peripheral blood buffy coat cells obtained by leukapheresis from the marrow donor on days 1 and 2, at a median dose of 2.3 (range, 1.2 to 6.9) ×108 TNC/kg to ensure consistent hematopoietic engraftment. MTX, at a dose of 0.4 mg/kg intravenously is used as postgrafting immunosuppression and administered on days +1, +3, +6 and +11, then weekly thereafter until day 102. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 673.1±65.0 °C at 760 mmHg |
| Melting Point | 220-225ºC |
| Molecular Formula | C11H16N4O4 |
| Molecular Weight | 268.269 |
| Flash Point | 360.9±34.3 °C |
| Exact Mass | 268.117157 |
| PSA | 112.13000 |
| LogP | -2.16 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.793 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | UN 2811 6.1 / PGIII |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Diclofenac toxicity in human intestine ex vivo is not related to the formation of intestinal metabolites.
Arch. Toxicol. 89(1) , 107-19, (2015) The use of diclofenac (DCF), a nonsteroidal anti-inflammatory drug, is associated with a high prevalence of gastrointestinal side effects. In vivo studies in rodents suggested that reactive metabolite... |
|
|
Suppression of Cpn10 increases mitochondrial fission and dysfunction in neuroblastoma cells.
PLoS ONE 9(11) , e112130, (2014) To date, several regulatory proteins involved in mitochondrial dynamics have been identified. However, the precise mechanism coordinating these complex processes remains unclear. Mitochondrial chapero... |
|
|
The HMGB1 protein sensitizes colon carcinoma cells to cell death triggered by pro-apoptotic agents.
Int. J. Oncol. 46(2) , 667-76, (2014) The HMGB1 protein has multiple functions in tumor biology and can act both as a transcription factor and as a cytokine. HMGB1 is released during cell death, and in our previous studies we demonstrated... |
| Nipent |
| DCF |
| yk-176 |
| (R)-3-(2-Deoxy-b-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol |
| Imidazo(4,5-d)(1,3)diazepin-8-ol, 3-(2-deoxy-β-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydro-, (R)- |
| Imidazo[4,5-d][1,3]diazepin-8-ol, 3- (2-deoxy-β-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydro-, (R)- |
| (8R)-3-(2-Deoxy-β-D-erythro-pentofuranosyl)-3,4,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol |
| Imidazo[4,5-d][1,3]diazepin-8-ol, 3-(2-deoxy-β-D-erythro-pentofuranosyl)-3,4,7,8-tetrahydro-, (R)- |
| deoxycoformycin |
| 2'-deoxy-D-coformycin |
| Pentostatin |
| 2'-deoxycoformycin |
| (R)-3-(2-Deoxy-β-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo(4,5-d)(1,3)diazepin-8-ol |
| Cl 825 |
| CO-Vidarabine |
| Co-V |
| ci-825 |
| Imidazo[4,5-d][1,3]diazepin-8-ol, 3- (2-deoxy-β-D-erythro-pentofuranosyl)-3,6,7,8- tetrahydro-, (R)- |
| (8R)-3-[(2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol |
| Imidazo[4,5-d][1,3]diazepin-8-ol, 3-(2-deoxy-β-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydro-, (8R)- |
| PD-ADI |
| Imidazo(4,5-d)(1,3)diazepin-8-ol, 3-(2-deoxy-β-D-erythro-pentofuranosyl)-3,4,7,8-tetrahydro-, (R)- |
| (8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxyméthyl)tétrahydrofuran-2-yl]-3,6,7,8-tétrahydroimidazo[4,5-d][1,3]diazépin-8-ol |
| Coforin |
![2-amino-1-[5-amino-1-(phenylmethyl)-1h-imidazol-4-yl] ethanone Structure](https://image.chemsrc.com/caspic/363/69195-91-1.png) CAS#:69195-91-1
CAS#:69195-91-1 CAS#:69195-97-7
CAS#:69195-97-7![3-(2-deoxy-3,5-di-O-p-toluoyl-α-D-erythro-pentofuranosyl)-6,7-dihydroimidazo[4,5-d][1,3]diazepin-8(3H)-one Structure](https://image.chemsrc.com/caspic/362/69196-02-7.png) CAS#:69196-02-7
CAS#:69196-02-7 CAS#:69195-92-2
CAS#:69195-92-2
