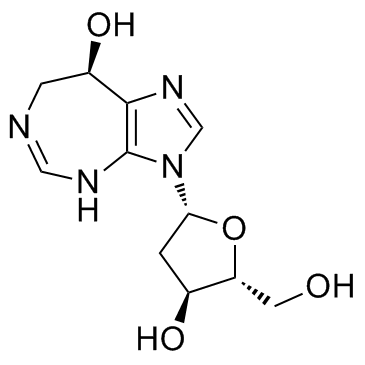
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
NI2931000
-
CHEMICAL NAME :
-
Imidazo(4,5-d)(1,3)diazepin-8-ol, 3-(2-deoxy-beta-D-pentofuranosyl)-3,6,7,8-tetrahydro- , (R)-
-
CAS REGISTRY NUMBER :
-
53910-25-1
-
LAST UPDATED :
-
199703
-
DATA ITEMS CITED :
-
23
-
MOLECULAR FORMULA :
-
C11-H16-N4-O4
-
MOLECULAR WEIGHT :
-
268.31
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
390 mg/kg/3D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Blood - thrombocytopenia Blood - other hemolysis with or without anemia
-
REFERENCE :
-
IEDIEP Internal Medicine. (The Japanese Society of Internal Medicine, 34-3, 3-chome, Hongo, Bunkyo-ku, Tokyo 113, Japan) V.31- 1992- Volume(issue)/page/year: 34,593,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
227 mg/kg
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - hematuria Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 24,7888,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
122 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB84-211424
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
360 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - miosis (pupillary constriction) Gastrointestinal - changes in structure or function of salivary glands Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 24,7888,1990 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
650 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Endocrine - other changes Blood - changes in bone marrow (not otherwise specified)
-
REFERENCE :
-
TOPADD Toxicologic Pathology. (c/o Dr. F.A. de la Iglesia, Warner-Lambert Co., Pharmaceutical Research Div., POB 1047, Ann Arbor, MI 48106) V.6(3/4)- 1978- Volume(issue)/page/year: 22,519,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
80 mg/kg/4W-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - changes in lung weight Endocrine - changes in spleen weight Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
CCPHDZ Cancer Chemotherapy and Pharmacology. (Springer-Verlag New York, Inc., Service Center, 44 Hartz Way, Secaucus, NJ 07094) V.1- 1978- Volume(issue)/page/year: 5,83,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
18200 ug/kg/26W-C
-
TOXIC EFFECTS :
-
Blood - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,4277,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
28 mg/kg/4W-I
-
TOXIC EFFECTS :
-
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Blood - changes in leukocyte (WBC) count Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,1271,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
91 ug/kg/26W-C
-
TOXIC EFFECTS :
-
Blood - other changes Nutritional and Gross Metabolic - weight loss or decreased weight gain Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,4277,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
14 mg/kg/4W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume increased Kidney, Ureter, Bladder - other changes in urine composition Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,1271,1991 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
7500 ug/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 44,325,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
7500 ug/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - sex ratio
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 44,325,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
500 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - blood and lymphatic systems (including spleen and marrow) Reproductive - Specific Developmental Abnormalities - immune and reticuloendothelial system
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 25(2),36A,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 40,615,1989
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 45,91,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 47,17,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 47,17,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,3977,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
8300 ug/kg
-
SEX/DURATION :
-
male 9 week(s) pre-mating female 2 week(s) pre-mating - 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,3965,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
8300 ug/kg
-
SEX/DURATION :
-
male 9 week(s) pre-mating female 2 week(s) pre-mating - 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,3965,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
28 ug/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,4329,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
28 ug/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,4329,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
1300 ug/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Fertility - abortion Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 25,4319,1991
|



![2-amino-1-[5-amino-1-(phenylmethyl)-1h-imidazol-4-yl] ethanone structure](https://image.chemsrc.com/caspic/363/69195-91-1.png)

![3-(2-deoxy-3,5-di-O-p-toluoyl-α-D-erythro-pentofuranosyl)-6,7-dihydroimidazo[4,5-d][1,3]diazepin-8(3H)-one structure](https://image.chemsrc.com/caspic/362/69196-02-7.png)