(15S,16E)-16,17,20,21-Tetradehydro-16-formyl-18,19-secoyohimban-19-oic acid methyl ester
Modify Date: 2024-02-08 19:00:35
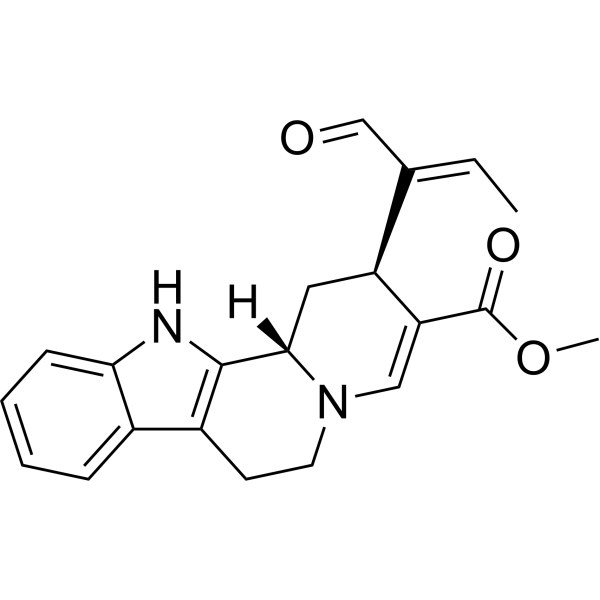
(15S,16E)-16,17,20,21-Tetradehydro-16-formyl-18,19-secoyohimban-19-oic acid methyl ester structure
|
Common Name | (15S,16E)-16,17,20,21-Tetradehydro-16-formyl-18,19-secoyohimban-19-oic acid methyl ester | ||
|---|---|---|---|---|
| CAS Number | 5523-37-5 | Molecular Weight | 350.41 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C21H22N2O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of (15S,16E)-16,17,20,21-Tetradehydro-16-formyl-18,19-secoyohimban-19-oic acid methyl esterVallesiachotamine, a known monoterpene indole alkaloid, possesses anti-tumor activity[1]. |
| Name | methyl (2S)-2-[(E)-1-oxobut-2-en-2-yl]-1,2,6,7,12,12b-hexahydroindolo[2,3-a]quinolizine-3-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Description | Vallesiachotamine, a known monoterpene indole alkaloid, possesses anti-tumor activity[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Vallesiachotamine induces G0/G1 arrest and increased the proportion of sub-G1 hypodiploid cells (at 11 μM and 22 μM) and this effect is not dependent on time of incubation. Vallesiachotamine acts by promoting G0/G1 cell cycle arrest, apoptosis and necrosis[1]. |
| References |
| Molecular Formula | C21H22N2O3 |
|---|---|
| Molecular Weight | 350.41 |
| Exact Mass | 350.16300 |
| PSA | 62.40000 |
| LogP | 3.22700 |
| 18,19-Secoyohimban-19-oic acid,16,17,20,21-tetradehydro-16-formyl-,methyl ester,(15beta,16E) |