Schizandrin B
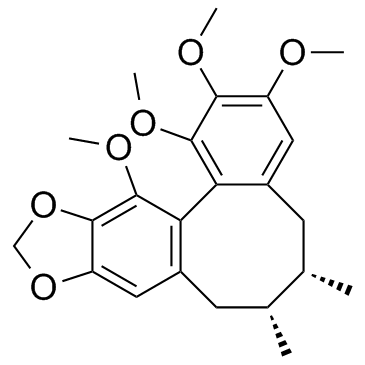
Schizandrin B structure
|
Common Name | Schizandrin B | ||
|---|---|---|---|---|
| CAS Number | 61281-37-6 | Molecular Weight | 400.465 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 545.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C23H28O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 220.4±30.0 °C | |
Use of Schizandrin BSchisandrin B(Wuweizisu-B) is a dibenzocyclooctadiene derivative isolated from Fructus Schisandrae, has been shown to produce antioxidant effect on rodent liver and heart.IC50 value:Target: in vitro: Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-κB. SB inhibited mitogen-induced proliferation and cytokine secretion by lymphocytes [1]. Sch B can protect neuronal cells against oxidative challenge, presumably by functioning as a hormetic agent to sustain cellular redox homeostasis and mitoenergetic capacity in neuronal cells [2]. Sch B exerted significant neuroprotective effects against microglial-mediated inflammatory injury in microglia-neuron co-cultures. Sch B significantly downregulated pro-inflammatory cytokines, including nitrite oxide (NO), tumor necrosis factor (TNF)-α, prostaglandin E(2) (PGE(2)), interleukin (IL)-1β and IL-6 [3]. Sch B could inhibit TGF-β induced EMT of 4T1 cells and of primary human breast cancer cells [4].in vivo: Similar anti-inflammatory effects of SB on lymphocyte proliferation and cytokine secretion were also observed in vivo [1]. Treatment with Sch B in CsA-treated mice significantly suppressed the elevation of blood urea nitrogen (BUN) and serum creatinine levels and attenuated the histopathological changes. Additionally, Sch B also decreased renal MDA levels and increased GSH levels in CsA-treated mice [5]. |
| Name | Schisandrin B |
|---|---|
| Synonym | More Synonyms |
| Description | Schisandrin B(Wuweizisu-B) is a dibenzocyclooctadiene derivative isolated from Fructus Schisandrae, has been shown to produce antioxidant effect on rodent liver and heart.IC50 value:Target: in vitro: Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-κB. SB inhibited mitogen-induced proliferation and cytokine secretion by lymphocytes [1]. Sch B can protect neuronal cells against oxidative challenge, presumably by functioning as a hormetic agent to sustain cellular redox homeostasis and mitoenergetic capacity in neuronal cells [2]. Sch B exerted significant neuroprotective effects against microglial-mediated inflammatory injury in microglia-neuron co-cultures. Sch B significantly downregulated pro-inflammatory cytokines, including nitrite oxide (NO), tumor necrosis factor (TNF)-α, prostaglandin E(2) (PGE(2)), interleukin (IL)-1β and IL-6 [3]. Sch B could inhibit TGF-β induced EMT of 4T1 cells and of primary human breast cancer cells [4].in vivo: Similar anti-inflammatory effects of SB on lymphocyte proliferation and cytokine secretion were also observed in vivo [1]. Treatment with Sch B in CsA-treated mice significantly suppressed the elevation of blood urea nitrogen (BUN) and serum creatinine levels and attenuated the histopathological changes. Additionally, Sch B also decreased renal MDA levels and increased GSH levels in CsA-treated mice [5]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 545.0±50.0 °C at 760 mmHg |
| Molecular Formula | C23H28O6 |
| Molecular Weight | 400.465 |
| Flash Point | 220.4±30.0 °C |
| Exact Mass | 400.188599 |
| PSA | 55.38000 |
| LogP | 6.46 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.543 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| HS Code | 2942000000 |
|
~% 
Schizandrin B CAS#:61281-37-6 |
| Literature: Heterocycles, , vol. 37, # 2 p. 739 - 742 |
|
~% 
Schizandrin B CAS#:61281-37-6 |
| Literature: Heterocycles, , vol. 37, # 2 p. 739 - 742 |
|
~% 
Schizandrin B CAS#:61281-37-6 |
| Literature: Heterocycles, , vol. 37, # 2 p. 739 - 742 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2942000000 |
|---|
| Benzo[3',4']cycloocta[1',2':4,5]benzo[1,2-d][1,3]dioxole, 5,6,7,8-tetrahydro-1,2,3,13-tetramethoxy-6,7-dimethyl- |
| MFCD00210555 |
| GEMA-SCHIZANDRIN |
| 1,2,3,13-Tetramethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3',4']cycloocta[1',2':4,5]benzo[1,2-d][1,3]dioxole |
| Schisandrin B |