Br-C10-methyl ester
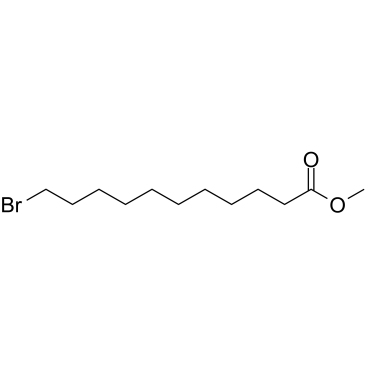
Br-C10-methyl ester structure
|
Common Name | Br-C10-methyl ester | ||
|---|---|---|---|---|
| CAS Number | 6287-90-7 | Molecular Weight | 279.21400 | |
| Density | 1.157 g/mL at 25ºC(lit.) | Boiling Point | 115ºC0.04 mm Hg(lit.) | |
| Molecular Formula | C12H23BrO2 | Melting Point | 13°C(lit.) | |
| MSDS | USA | Flash Point | >230 °F | |
Use of Br-C10-methyl esterBr-C10-methyl ester is a PROTAC linker, which refers to the alkyl/ether composition. Br-C10-methyl ester is used in the synthesis of a series of PROTACs (MS432). PROTACs contain two different ligands connected by a linker; one is the VHL ligand portion and the other is for the target protein[1]. |
| Name | methyl 11-bromoundecanoate |
|---|---|
| Synonym | More Synonyms |
| Description | Br-C10-methyl ester is a PROTAC linker, which refers to the alkyl/ether composition. Br-C10-methyl ester is used in the synthesis of a series of PROTACs (MS432). PROTACs contain two different ligands connected by a linker; one is the VHL ligand portion and the other is for the target protein[1]. |
|---|---|
| Related Catalog | |
| Target |
Alkyl/ether |
| References |
| Density | 1.157 g/mL at 25ºC(lit.) |
|---|---|
| Boiling Point | 115ºC0.04 mm Hg(lit.) |
| Melting Point | 13°C(lit.) |
| Molecular Formula | C12H23BrO2 |
| Molecular Weight | 279.21400 |
| Flash Point | >230 °F |
| Exact Mass | 278.08800 |
| PSA | 26.30000 |
| LogP | 4.06520 |
| Index of Refraction | n20/D 1.465(lit.) |
| Personal Protective Equipment | Eyeshields;Gloves |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2915900090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2915900090 |
|---|---|
| Summary | 2915900090 other saturated acyclic monocarboxylic acids and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:5.5% General tariff:30.0% |
|
Versatile synthesis of phenoxydiazirine-based fatty acid analogues and photoreactive galactosylceramide.
Bioorg. Med. Chem. Lett. 12(1) , 89-91, (2002) A versatile synthesis of diazirine-based photoreactive fatty acid analogues is reported. The key step is phenoxy alkylation of diazirine with halo alkyl acid esters. The conditions described will be a... |
|
|
Studies on ω-Oxidation of Fatty Acids in vitro. KAMEI S, et al.
J. Biochem. 56(1) , 72-76, (1964)
|
|
|
A Three Step Synthesis of 11-Cycloheptylundecanoic Acid, a Component of the Thermoacidophile Alicyclobacillus cycloheptanicus. Hassarajani SA and Mamdapur VR.
Molecules 3(2) , 41-43, (1998)
|
| MFCD00045047 |
| methyl 11-bromoundecan-1-oate |
| 11-bromo-undecanoic acid methyl ester |
| 11-Brom-undecansaeure-methylester |
| methyl 11-bromoundecanate |
| 11-Brom-undecylsaeure-methylester |
 CAS#:67-56-1
CAS#:67-56-1 CAS#:2834-05-1
CAS#:2834-05-1 CAS#:111-81-9
CAS#:111-81-9 CAS#:15949-84-5
CAS#:15949-84-5 CAS#:186581-53-3
CAS#:186581-53-3 CAS#:24724-07-0
CAS#:24724-07-0 CAS#:112-38-9
CAS#:112-38-9 CAS#:57467-59-1
CAS#:57467-59-1 CAS#:74198-06-4
CAS#:74198-06-4 CAS#:109023-44-1
CAS#:109023-44-1 CAS#:56683-54-6
CAS#:56683-54-6 CAS#:143814-66-8
CAS#:143814-66-8 CAS#:1731-86-8
CAS#:1731-86-8 CAS#:3669-80-5
CAS#:3669-80-5 CAS#:106-02-5
CAS#:106-02-5![[(11-Iodoundecyl)oxy]trimethylsilane structure](https://image.chemsrc.com/caspic/404/26305-84-0.png) CAS#:26305-84-0
CAS#:26305-84-0 CAS#:26305-83-9
CAS#:26305-83-9 CAS#:34010-21-4
CAS#:34010-21-4 CAS#:71310-21-9
CAS#:71310-21-9