Fenretinide
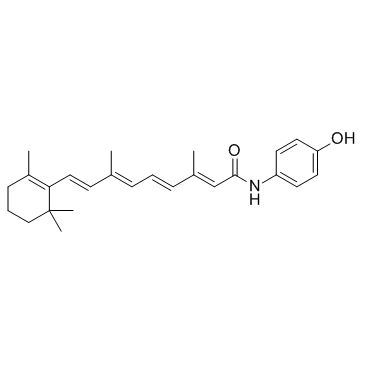
Fenretinide structure
|
Common Name | Fenretinide | ||
|---|---|---|---|---|
| CAS Number | 65646-68-6 | Molecular Weight | 391.546 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 597.6±42.0 °C at 760 mmHg | |
| Molecular Formula | C26H33NO2 | Melting Point | 162-163°C | |
| MSDS | Chinese USA | Flash Point | 315.2±27.9 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of FenretinideFenretinide is a synthetic retinoid deriverative, binding to the retinoic acid receptors (RAR) at concentrations necessary to induce cell death. |
| Name | 4-hydroxyphenyl retinamide |
|---|---|
| Synonym | More Synonyms |
| Description | Fenretinide is a synthetic retinoid deriverative, binding to the retinoic acid receptors (RAR) at concentrations necessary to induce cell death. |
|---|---|
| Related Catalog | |
| In Vitro | Fenretinide exerts not just acute but also long term antitumor activity in selected T-ALL cell lines. Fenretinide inhibits DES activity in CCRF-CEM leukemia cells in a dose and time dependent manner, leading to a concomitant increase of the endogenous cellular dhCer content. Fenretinide (3 μM)-induced dhCer accumulation in both CCRF-CEM and Jurkat cells[1]. Ceramide inhibition with fenretinide protects insulin signaling. Fenretinide prevents lipid-induced reductions in insulin-stimulated glucose uptake[2]. Fenretinide inhibits OVCAR-5 cell proliferation and viability at concentrations higher than 1 microM, with 70-90% growth inhibition at 10 microM. Fenretinide (1 microM) significantly inhibits OVCAR-5 invasion after 3 days preincubation. Endothelial cells treated with 1 microM 4-HPR fails to form tubes, but forms small cellular aggregates[4]. |
| In Vivo | Fenretinide (10 mg/kg, i.p.) selectively inhibits ceramide accumulation HFD-fed male C57Bl/6 mice. Fenretinide treatment improves glucose tolerance and insulin sensitivity as determined by both glucose and insulin tolerance tests[2]. Addition of 25 mg/kg ketoconazole to Fenretinide in NOD/SCID mice increased 4-HPR plasma levels[3]. |
| Cell Assay | Standard XTT assay is used to determine cell viability. For fenretinide-only treatments, cells are plated in 96-well plates at 750,000 cells/mL and 100 μL/well. After 4 h, treatments are added on 50 μL/well obtaining a final density of 500,000 cells/mL and final volume of 150 μL/well. Four replicates are used per experimental condition. XTT reagent mixture is added 4 h before the end of selected treatment period and absorbance at 490 nm is determined per each well. A slightly modified protocol is used for analysis of the effect of myriocin (final concentration of 100 nM) or antioxidant on Fenretinide treatment. Briefly, cells are seeded on 60 mm culture dishes and myriocin or antioxidants added after 4 h. Fenretinide treatment is added 2 h later and cells are plated in quadruplicates in 96 well plates (150 μL/well). |
| Animal Admin | Male mice (C57Bl6) are fed a standard chow or a high-fat diet (HFD) from 5 to 17 weeks, at which point half of the HFD-fed mice begin receiving fenretinide in drinking water for 4 weeks. Fenretinide is dissolved in 100% ethanol and diluted in water to 10 μg/mL. Control treatment water receives an equal amount of ethanol (0.5%). FEN water is prepared in low-light conditions and administered in light-protective bottles. Water is replaced every 1-2 days, and no precipitation of FEN is noted at any time. Animal weights are recorded at the beginning and end of the treatment period. Following a 4-week FEN treatment, mice undergo intraperitoneal glucose and insulin tolerance tests. For both tests, mice are fasted for 6 h andreceive an injection of either glucose (1 g/kg of body weight) or insulin (0.75 units/kg of body weight). Blood glucose is determined at the times indicated by the Bayer Contour® glucose meter, and insulin is measured with the rat/mouse insulin ELISA kit. The insulin resistance index is assessed by using fasting blood glucose and insulin levels to compute the homeostatic model assessment of insulin resistance (HOMA-IR), where a higher number represents greater insulin resistance. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 597.6±42.0 °C at 760 mmHg |
| Melting Point | 162-163°C |
| Molecular Formula | C26H33NO2 |
| Molecular Weight | 391.546 |
| Flash Point | 315.2±27.9 °C |
| Exact Mass | 391.251129 |
| PSA | 49.33000 |
| LogP | 7.41 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.607 |
| Storage condition | −20°C |
| Stability | Light Sensitive - Protect from Light Exposure |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302 + H312 + H332-H315-H319-H335-H360 |
| Precautionary Statements | P201-P261-P280-P305 + P351 + P338-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R60;R61;R20/21/22;R36/37/38 |
| Safety Phrases | S53-S26-S36/37/39-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | VH6420000 |
|
~82% 
Fenretinide CAS#:65646-68-6 |
| Literature: Sangmam, Charles; Winum, Jean-Yves; Lucas, Marc; Montero, Jean-Louis; Chavis, Claude Synthetic Communications, 1998 , vol. 28, # 16 p. 2945 - 2958 |
|
~% 
Fenretinide CAS#:65646-68-6 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 73, # 6 p. 745 - 751 |
|
~% 
Fenretinide CAS#:65646-68-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 17, # 3 p. 836 - 840 |
|
Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes.
PLoS ONE 10(2) , e0115686, (2015) Antimetabolites are a class of effective anticancer drugs interfering in essential biochemical processes. 5-Fluorouracil (5-FU) and its prodrug Capecitabine are widely used in the treatment of several... |
|
|
Enhanced killing of SCC17B human head and neck squamous cell carcinoma cells after photodynamic therapy plus fenretinide via the de novo sphingolipid biosynthesis pathway and apoptosis.
Int. J. Oncol. 46(5) , 2003-10, (2015) Because photodynamic therapy (PDT) alone is not always effective as an anticancer treatment, PDT is combined with other anticancer agents for improved efficacy. The clinically-relevant fenretinide [N-... |
|
|
Modification of sphingolipid metabolism by tamoxifen and N-desmethyltamoxifen in acute myelogenous leukemia--Impact on enzyme activity and response to cytotoxics.
Biochim. Biophys. Acta 1851 , 919-28, (2015) The triphenylethylene antiestrogen, tamoxifen, can be an effective inhibitor of sphingolipid metabolism. This off-target activity makes tamoxifen an interesting ancillary for boosting the apoptosis-in... |
| 4-Hydroxyphenyl retinamide |
| Fenretinide |
| 4-HPR |
| 15-[(4-Hydroxyphenyl)amino]retinal |
| fenretinida |
| Retinal, 15-[(4-hydroxyphenyl)amino]- |
| fenretinidum |
| MFCD00792674 |


![[4-[[(2Z,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoyl]amino]phenyl] 2,2-dimethylpropanoate structure](https://image.chemsrc.com/caspic/492/75664-77-6.png) CAS#:75664-77-6
CAS#:75664-77-6