Sulbactam
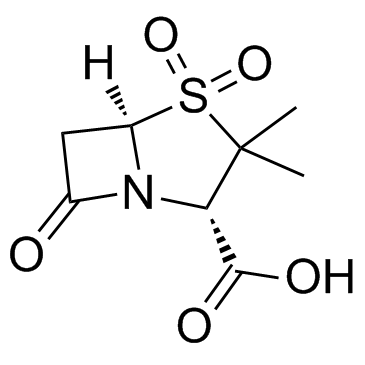
Sulbactam structure
|
Common Name | Sulbactam | ||
|---|---|---|---|---|
| CAS Number | 68373-14-8 | Molecular Weight | 233.242 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 567.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C8H11NO5S | Melting Point | 146-151ºC | |
| MSDS | Chinese USA | Flash Point | 297.1±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of SulbactamSulbactam(Betamaze) is an irreversible β-lactamase inhibitor.Target: β-lactamase; AntibacterialSulbactam is a mechanism-based inhibitor of beta-lactamase enzymes used in clinical practice. sulbactam was the antimicrobial agent responsible for the killing of these organisms [1]. sulbactam may prove effective for non-life-threatening A. baumannii infections. Its role in the treatment of severe infections is unknown. However, the current formulation of sulbactam alone may allow its use at higher doses and provide new potential synergic combinations, particularly for those infections by A. baumannii resistant to imipenem [2]. |
| Name | sulbactam |
|---|---|
| Synonym | More Synonyms |
| Description | Sulbactam(Betamaze) is an irreversible β-lactamase inhibitor.Target: β-lactamase; AntibacterialSulbactam is a mechanism-based inhibitor of beta-lactamase enzymes used in clinical practice. sulbactam was the antimicrobial agent responsible for the killing of these organisms [1]. sulbactam may prove effective for non-life-threatening A. baumannii infections. Its role in the treatment of severe infections is unknown. However, the current formulation of sulbactam alone may allow its use at higher doses and provide new potential synergic combinations, particularly for those infections by A. baumannii resistant to imipenem [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 567.7±50.0 °C at 760 mmHg |
| Melting Point | 146-151ºC |
| Molecular Formula | C8H11NO5S |
| Molecular Weight | 233.242 |
| Flash Point | 297.1±30.1 °C |
| Exact Mass | 233.035797 |
| PSA | 100.13000 |
| LogP | -1.39 |
| Vapour Pressure | 0.0±3.4 mmHg at 25°C |
| Index of Refraction | 1.605 |
| Storage condition | -20°C |
| Precursor 10 | |
|---|---|
| DownStream 2 | |
| HS Code | 2941109900 |
|---|
|
Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii.
Antimicrob. Agents Chemother. 59(3) , 1680-9, (2015) Sulbactam is a class A β-lactamase inhibitor with intrinsic whole-cell activity against certain bacterial species, including Acinetobacter baumannii. The clinical use of sulbactam for A. baumannii inf... |
|
|
The spread of bla OXA-48 and bla OXA-244 carbapenemase genes among Klebsiella pneumoniae, Proteus mirabilis and Enterobacter spp. isolated in Moscow, Russia.
Ann. Clin. Microbiol. Antimicrob. 14 , 46, (2015) The spread of carbapenemase-producing Enterobacteriaceae (CPE) is a great problem of healthcare worldwide. Study of the spread for bla OXA-48-like genes coding epidemically significant carbapenemases ... |
|
|
Investigation of a possible outbreak of carbapenem-resistant Acinetobacter baumannii in Odense, Denmark using PFGE, MLST and whole-genome-based SNPs.
J. Antimicrob. Chemother. 70 , 1965-8, (2015) The objectives were to study a possible outbreak of carbapenem-resistant Acinetobacter baumannii by comparing three different typing methods (PFGE, MLST and whole-genome SNPs) and to compare the resis... |
| penicillanic acid S,S-dioxide |
| 6,6-dihydropenicillanic acid S,S-dioxide |
| EINECS 269-878-2 |
| 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-, 4,4-dioxide, (2S-cis)- |
| 4,4-dioxopenicillanic acid |
| (2S-cis)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid 4,4-Dioxide |
| penicillanic Acid 1,1-Dioxide |
| DSSTox_CID_3605 |
| UNII-S4TF6I2330 |
| Sulbactam free acid |
| (2S,5R)-3,3-dimethyl-4,4,7-trioxo-4λ<sup>6</sup>-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-, 4,4-dioxide, (2S,5R)- |
| Penicillanic Acid Sulfone |
| Unacim |
| (2S,5R)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide |
| Sulbactamum |
| Sulbactam |
| acide (2S,5R)-3,3-diméthyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylique-4,4-dioxyde |
 CAS#:76646-91-8
CAS#:76646-91-8 CAS#:7439-96-5
CAS#:7439-96-5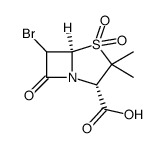 CAS#:810692-15-0
CAS#:810692-15-0 CAS#:7440-02-0
CAS#:7440-02-0![(2S,5R)-Benzyl 3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-Dioxide Structure](https://image.chemsrc.com/caspic/458/69388-78-9.png) CAS#:69388-78-9
CAS#:69388-78-9 CAS#:76517-16-3
CAS#:76517-16-3 CAS#:688-73-3
CAS#:688-73-3 CAS#:76517-25-4
CAS#:76517-25-4![(2S-cis)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Structure](https://image.chemsrc.com/caspic/217/87-53-6.png) CAS#:87-53-6
CAS#:87-53-6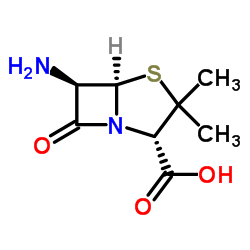 CAS#:551-16-6
CAS#:551-16-6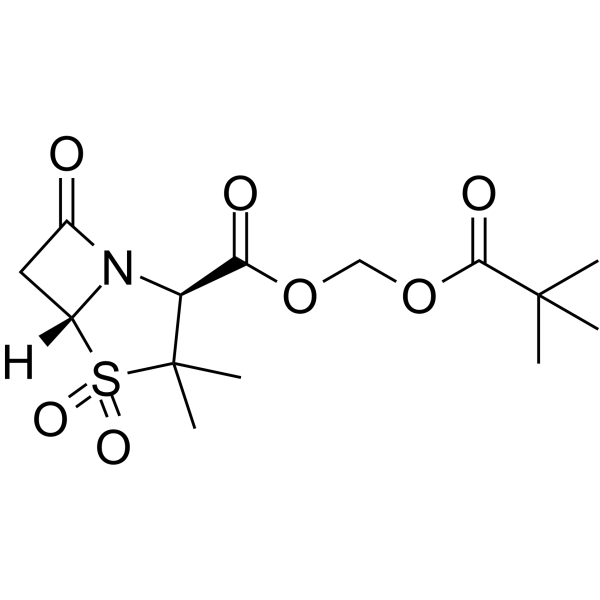 CAS#:69388-79-0
CAS#:69388-79-0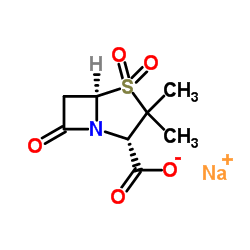 CAS#:69388-84-7
CAS#:69388-84-7