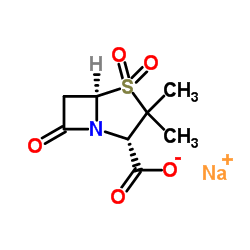
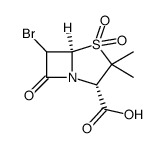
68373-14-8
| Name | sulbactam |
|---|---|
| Synonyms |
penicillanic acid S,S-dioxide
6,6-dihydropenicillanic acid S,S-dioxide EINECS 269-878-2 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-, 4,4-dioxide, (2S-cis)- 4,4-dioxopenicillanic acid (2S-cis)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid 4,4-Dioxide penicillanic Acid 1,1-Dioxide DSSTox_CID_3605 UNII-S4TF6I2330 Sulbactam free acid (2S,5R)-3,3-dimethyl-4,4,7-trioxo-4λ<sup>6</sup>-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-, 4,4-dioxide, (2S,5R)- Penicillanic Acid Sulfone Unacim (2S,5R)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide Sulbactamum Sulbactam acide (2S,5R)-3,3-diméthyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylique-4,4-dioxyde |
| Description | Sulbactam(Betamaze) is an irreversible β-lactamase inhibitor.Target: β-lactamase; AntibacterialSulbactam is a mechanism-based inhibitor of beta-lactamase enzymes used in clinical practice. sulbactam was the antimicrobial agent responsible for the killing of these organisms [1]. sulbactam may prove effective for non-life-threatening A. baumannii infections. Its role in the treatment of severe infections is unknown. However, the current formulation of sulbactam alone may allow its use at higher doses and provide new potential synergic combinations, particularly for those infections by A. baumannii resistant to imipenem [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 567.7±50.0 °C at 760 mmHg |
| Melting Point | 146-151ºC |
| Molecular Formula | C8H11NO5S |
| Molecular Weight | 233.242 |
| Flash Point | 297.1±30.1 °C |
| Exact Mass | 233.035797 |
| PSA | 100.13000 |
| LogP | -1.39 |
| Vapour Pressure | 0.0±3.4 mmHg at 25°C |
| Index of Refraction | 1.605 |
| Storage condition | -20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2941109900 |
| Precursor 10 | |
|---|---|
| DownStream 2 | |
| HS Code | 2941109900 |
|---|



![(2S,5R)-Benzyl 3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-Dioxide structure](https://image.chemsrc.com/caspic/458/69388-78-9.png)

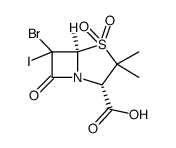
![(2S-cis)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid structure](https://image.chemsrc.com/caspic/217/87-53-6.png)