Triclabendazole

Triclabendazole structure
|
Common Name | Triclabendazole | ||
|---|---|---|---|---|
| CAS Number | 68786-66-3 | Molecular Weight | 359.658 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 495.9±55.0 °C at 760 mmHg | |
| Molecular Formula | C14H9Cl3N2OS | Melting Point | 175-176°C | |
| MSDS | Chinese USA | Flash Point | 253.7±31.5 °C | |
Use of TriclabendazoleTriclabendazole(CGA89317) is a benzimidazole, it binds to tubulin impairing intracellular transport mechanisms and interferes with protein synthesis.Target: Microtubule/TubulinTriclabendazole treatment produces percentage decreases of the fluke egg output by 15.3%, 4.3% and 36.6%, respectively, in sheep, dairy cows and heifers, these results indicate the presence of TCBZ-resistant Fasciola hepatica in sheep and cattle on this farm [1]. Triclabendazole sulphoxide (50 mg/mL) results in extensive damage to the tegument of triclabendazole-susceptible F. hepatica, whereas triclabendazole-resistant flukes shows only localized and relatively minor disruption of the tegument covering the spines [2].Triclabendazole is metabolized into a number of compounds, depending on the route of administration, plasma levels peak at 18-24 hours (Triclabendazole sulphoxide) and 36-48 hours (Triclabendazole sulphone), neither Triclabendazole nor any toher metabolites can be detected in plasma. Triclabendazole sulphoxide blocks the transport of secretory bodies from the cell body to the tegumental surface, the block occurs at the site of their formation by the Golgi complex in the cell body, in their movement through the cytoplasmic connections to the syncytium, and in their movement from the base to the apex of the syncytium. Triclabendazole binds to the colchicine binding site on the β-tubulin molecule and this has been used at the basis for evaluating the relative acitvity of Triclabendazole [3]. |
| Name | Triclabendazole |
|---|---|
| Synonym | More Synonyms |
| Description | Triclabendazole(CGA89317) is a benzimidazole, it binds to tubulin impairing intracellular transport mechanisms and interferes with protein synthesis.Target: Microtubule/TubulinTriclabendazole treatment produces percentage decreases of the fluke egg output by 15.3%, 4.3% and 36.6%, respectively, in sheep, dairy cows and heifers, these results indicate the presence of TCBZ-resistant Fasciola hepatica in sheep and cattle on this farm [1]. Triclabendazole sulphoxide (50 mg/mL) results in extensive damage to the tegument of triclabendazole-susceptible F. hepatica, whereas triclabendazole-resistant flukes shows only localized and relatively minor disruption of the tegument covering the spines [2].Triclabendazole is metabolized into a number of compounds, depending on the route of administration, plasma levels peak at 18-24 hours (Triclabendazole sulphoxide) and 36-48 hours (Triclabendazole sulphone), neither Triclabendazole nor any toher metabolites can be detected in plasma. Triclabendazole sulphoxide blocks the transport of secretory bodies from the cell body to the tegumental surface, the block occurs at the site of their formation by the Golgi complex in the cell body, in their movement through the cytoplasmic connections to the syncytium, and in their movement from the base to the apex of the syncytium. Triclabendazole binds to the colchicine binding site on the β-tubulin molecule and this has been used at the basis for evaluating the relative acitvity of Triclabendazole [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 495.9±55.0 °C at 760 mmHg |
| Melting Point | 175-176°C |
| Molecular Formula | C14H9Cl3N2OS |
| Molecular Weight | 359.658 |
| Flash Point | 253.7±31.5 °C |
| Exact Mass | 357.950104 |
| PSA | 63.21000 |
| LogP | 5.97 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.724 |
| Stability | Stability |
|
Triclabendazole
Revision number: 5
SAFETY DATA SHEET Section1. IDENTIFICATION Product name:Triclabendazole Revision number:5 Section2. HAZARDS IDENTIFICATION GHS classification PHYSICAL HAZARDSNot classified Not classified HEALTH HAZARDS ENVIRONMENTAL HAZARDSNot classified GHS label elements, including precautionary statements Pictograms or hazard symbolsNone No signal word Signal word Hazard statementsNone None Precautionary statements: Section3. COMPOSITION/INFORMATION ON INGREDIENTS Substance/mixture:Substance Components:Triclabendazole Percent:>98.0%(LC)(T) CAS Number:68786-66-3 Synonyms:5-Chloro-6-(2,3-dichlorophenoxy)-2-(methylthio)benzimidazole , 6-Chloro-5-(2,3- dichlorophenoxy)-2-(methylthio)benzimidazole C14H9Cl3N2OS Chemical Formula: Section4. FIRST AID MEASURES Inhalation:Remove victim to fresh air and keep at rest in a position comfortable for breathing. Get medical advice/attention if you feel unwell. Skin contact:Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower. If skin irritation or rash occurs: Get medical advice/attention. Eye contact:Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. Ingestion:Get medical advice/attention if you feel unwell. Rinse mouth. A rescuer should wear personal protective equipment, such as rubber gloves and air- Protection of first-aiders: tight goggles. Section5. FIRE-FIGHTING MEASURES Suitable extinguishingDry chemical, foam, water spray, carbon dioxide. media: Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to from the chemical:generate poisonous fume. Triclabendazole Section5. FIRE-FIGHTING MEASURES Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing method according to the surrounding situation is used. Uninvolved persons should evacuate to a safe place. In case of fire in the surroundings: Remove movable containers if safe to do so. Special protectiveWhen extinguishing fire, be sure to wear personal protective equipment. equipment for firefighters: Section6. ACCIDENTAL RELEASE MEASURES Use personal protective equipment. Keep people away from and upwind of spill/leak. Personal precautions, protective equipment and Entry to non-involved personnel should be controlled around the leakage area by emergency procedures: roping off, etc. Environmental precautions: Prevent product from entering drains. Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it. containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with up: appropriate laws and regulations. Section7. HANDLING AND STORAGE Precautions for safe handling Handling is performed in a well ventilated place. Wear suitable protective equipment. Technical measures: Prevent dispersion of dust. Wash hands and face thoroughly after handling. Use a local exhaust if dust or aerosol will be generated. Advice on safe handling: Avoid contact with skin, eyes and clothing. Conditions for safe storage, including any incompatibilities Storage conditions:Keep container tightly closed. Store in a cool and dark place. Store away from incompatible materials such as oxidizing agents. Packaging material:Comply with laws. Section8. EXPOSURE CONTROLS / PERSONAL PROTECTION Install a closed system or local exhaust as possible so that workers should not be Engineering controls: exposed directly. Also install safety shower and eye bath. Personal protective equipment Respiratory protection: Dust respirator. Follow local and national regulations. Hand protection:Protective gloves. Eye protection:Safety glasses. A face-shield, if the situation requires. Skin and body protection: Protective clothing. Protective boots, if the situation requires. Section9. PHYSICAL AND CHEMICAL PROPERTIES Physical state (20°C):Solid Form:Crystal- Powder Colour:White - Slightly pale yellow red Odour:No data available pH: No data available Melting point/freezing point:176°C No data available Boiling point/range: Flash point:No data available Flammability or explosive limits: No data available Lower: Upper:No data available No data available Relative density: Solubility(ies): No data available [Water] [Other solvents]No data available 5.35 Log Pow: Triclabendazole Section10. STABILITY AND REACTIVITY Chemical stability:Stable under proper conditions. Possibility of hazardous No special reactivity has been reported. reactions: Incompatible materials: Oxidizing agents Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides, Hydrogen products:chloride Section11. TOXICOLOGICAL INFORMATION orl-rat LD50:>8 g/kg Acute Toxicity: ihl-rat LC50:>500 mg/m3/4H skn-rat LD50:>4 g/kg Skin corrosion/irritation: No data available No data available Serious eye damage/irritation: Germ cell mutagenicity: cyt-ctl-lym 25 mg/L/48H sce-ctl-lym 25 mg/L/48H Carcinogenicity: IARC =No data available No data available NTP = Reproductive toxicity:No data available DD6747000 RTECS Number: Section12. ECOLOGICAL INFORMATION Ecotoxicity: Fish:No data available Crustacea:No data available Algae:No data available Persistence / degradability: No data available BioaccumulativeNo data available potential(BCF): Mobility in soil Log Pow:5.35 Soil adsorption (Koc):No data available Henry's LawNo data available constant(PaM3/mol): Section13. DISPOSAL CONSIDERATIONS Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when disposing of the substance. Section14. TRANSPORT INFORMATION Hazards Class:Does not correspond to the classification standard of the United Nations UN-No:Not listed Section15. REGULATORY INFORMATION Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002 and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were prescribed. Triclabendazole SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Statements | H413 |
|---|---|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DD6747000 |
| HS Code | 2933990090 |
|
~80% 
Triclabendazole CAS#:68786-66-3 |
| Literature: SEQUENT SCIENTIFIC LIMITED; Rane, Ramkrishna Appaji; Verma, Sudhakar; Arulmoli, Thangavel Patent: US2013/303781 A1, 2013 ; Location in patent: Paragraph 0031 ; |
|
~65%
Detail
|
| Literature: Bulletin des Societes Chimiques Belges, , vol. 99, # 9 p. 673 - 701 |
|
~% 
Triclabendazole CAS#:68786-66-3 |
| Literature: US2013/303781 A1, ; |
|
~% 
Triclabendazole CAS#:68786-66-3 |
| Literature: US2013/303781 A1, ; |
|
~% 
Triclabendazole CAS#:68786-66-3 |
| Literature: US2013/303781 A1, ; |
|
~% 
Triclabendazole CAS#:68786-66-3 |
| Literature: US2013/303781 A1, ; |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Time-course and accumulation of triclabendazole and its metabolites in bile, liver tissues and flukes collected from treated sheep.
Exp. Parasitol. 136 , 14-9, (2014) The flukicidal compound triclabendazole (TCBZ) has a complex metabolic pattern that includes the systemic presence of its sulphoxide (TCBZ.SO) and sulphone (TCBZ.SO2) metabolites, usually recovered fr... |
|
|
The anthelmintic triclabendazole and its metabolites inhibit the membrane transporter ABCG2/BCRP.
Antimicrob. Agents Chemother. 56(7) , 3535-43, (2012) ABCG2/BCRP is an ATP-binding cassette transporter that extrudes compounds from cells in the intestine, liver, kidney, and other organs, such as the mammary gland, affecting pharmacokinetics and milk s... |
|
|
Isothermal microcalorimetry to study the activity of triclabendazole and its metabolites on juvenile and adult Fasciola hepatica.
Exp. Parasitol. 133(3) , 265-8, (2013) Isothermal microcalorimetry (IMC) is an analytical tool that continuously measures the heat flow generated by chemical, physical or biological processes. We have demonstrated that IMC is a useful tool... |
| Fasinex |
| MFCD00864519 |
| Triclabendazole |
| 6-Chloro-5-(2,3-dichlorophenoxy)-2-methylthio-benzimidazole |
| 5-Chloro-6-(2,3-dichlorophenoxy)-2-(methylsulfanyl)-1H-benzimidazole |
| 1H-Benzimidazole, 6-chloro-5-(2,3-dichlorophenoxy)-2-(methylthio)- |
| 5-Chloro-6-(2,3-dichlorophenoxy)-2-methylthio-1H-benzimidazole |




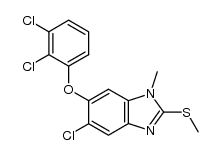
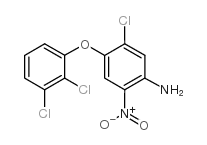
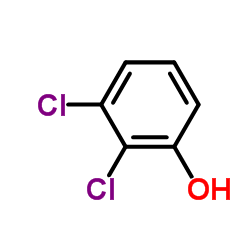


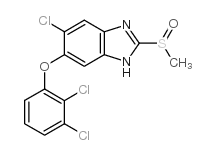 CAS#:100648-13-3
CAS#:100648-13-3
