CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XH8350000
-
CHEMICAL NAME :
-
4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(2-amino-2-phenylacetamido)-3,3- dimethyl-7-oxo-, D-(-)-
-
CAS REGISTRY NUMBER :
-
69-53-4
-
LAST UPDATED :
-
199806
-
DATA ITEMS CITED :
-
31
-
MOLECULAR FORMULA :
-
C16-H19-N3-O4-S
-
MOLECULAR WEIGHT :
-
349.44
-
WISWESSER LINE NOTATION :
-
T45 ANV ESTJ CMVYZR& F1 F1 GVQ -D
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
400 mg/kg/4W-I
-
TOXIC EFFECTS :
-
Blood - agranulocytosis Blood - other changes Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
160 mg/kg/4D-I
-
TOXIC EFFECTS :
-
Blood - agranulocytosis Blood - thrombocytopenia
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6200 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Parenteral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3250 mg/kg
-
TOXIC EFFECTS :
-
Brain and Coverings - recordings from specific areas of CNS Behavioral - somnolence (general depressed activity)
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4600 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intracerebral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
380 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - excitement Musculoskeletal - changes in teeth and supporting structures
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Parenteral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
28 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
>100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
7500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2500 mg/kg
-
SEX/DURATION :
-
female 4-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death
MUTATION DATA
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TEST SYSTEM :
-
Human Lymphocyte
-
DOSE/DURATION :
-
28 mg/L
-
REFERENCE :
-
HUTODJ Human Toxicology. (Macmillan Press Ltd., Houndmills, Basingstoke, Hants., RG 21 2XS, UK) V.1- 1981- Volume(issue)/page/year: 3,173,1984 *** REVIEWS *** IARC Cancer Review:Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 50,153,1990 IARC Cancer Review:Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 50,153,1990 IARC Cancer Review:Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 50,153,1990 *** OCCUPATIONAL EXPOSURE LIMITS *** OEL-RUSSIA:STEL 0.1 mg/m3 JAN 1993 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 81707 No. of Facilities: 1320 (estimated) No. of Industries: 2 No. of Occupations: 4 No. of Employees: 2942 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 81707 No. of Facilities: 1250 (estimated) No. of Industries: 2 No. of Occupations: 13 No. of Employees: 42346 (estimated) No. of Female Employees: 32858 (estimated)
|





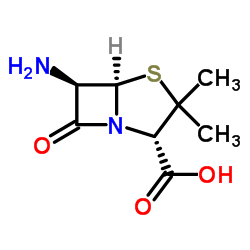 CAS#:551-16-6
CAS#:551-16-6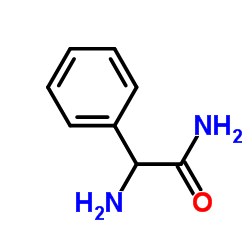 CAS#:700-63-0
CAS#:700-63-0 CAS#:24461-61-8
CAS#:24461-61-8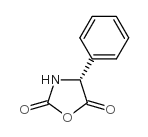 CAS#:3412-49-5
CAS#:3412-49-5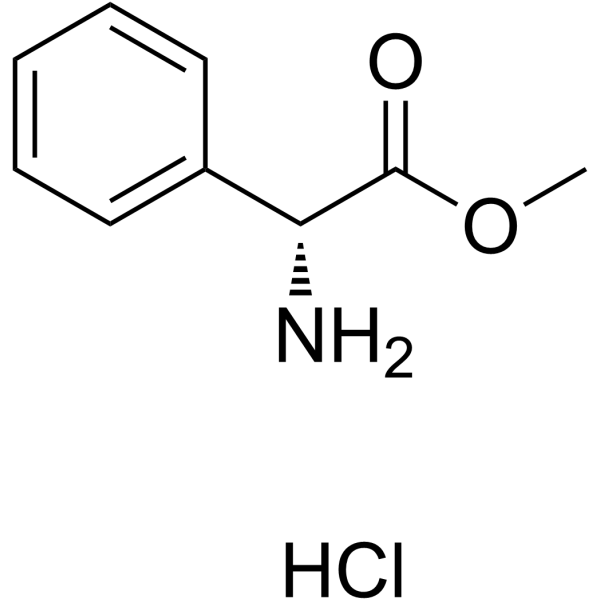 CAS#:19883-41-1
CAS#:19883-41-1 CAS#:15028-40-7
CAS#:15028-40-7 CAS#:33817-20-8
CAS#:33817-20-8 CAS#:37661-08-8
CAS#:37661-08-8 CAS#:37091-66-0
CAS#:37091-66-0 CAS#:875-74-1
CAS#:875-74-1![4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylicacid, 6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-,1,3-dihydro-3-oxo-1-isobenzofuranyl ester, (2S,5R,6R)- structure](https://image.chemsrc.com/caspic/262/47747-56-8.png) CAS#:47747-56-8
CAS#:47747-56-8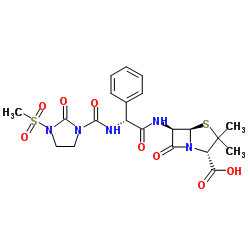 CAS#:51481-65-3
CAS#:51481-65-3 CAS#:61-33-6
CAS#:61-33-6 CAS#:1252608-05-1
CAS#:1252608-05-1 CAS#:611-73-4
CAS#:611-73-4
