1-Naphthohydroxamic acid
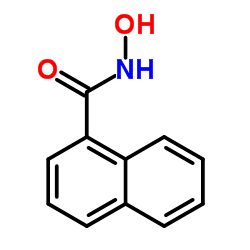
1-Naphthohydroxamic acid structure
|
Common Name | 1-Naphthohydroxamic acid | ||
|---|---|---|---|---|
| CAS Number | 6953-61-3 | Molecular Weight | 187.195 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C11H9NO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of 1-Naphthohydroxamic acid1-Naphthohydroxamic acid (Compound 2) is a potent and selective HDAC8 inhibitor with an IC50 of 14 μM. 1-Naphthohydroxamic acid is more selectively for HDAC8 than class I HDAC1 and class II HDAC6 (IC50 >100 μM). 1-Naphthohydroxamic acid does not increase global histone H4 acetylation and also does not reduce total intracellular HDAC activity[1][2].1-Naphthohydroxamic acid can induce tubulin acetylation[3]. |
| Name | N-hydroxynaphthalene-1-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | 1-Naphthohydroxamic acid (Compound 2) is a potent and selective HDAC8 inhibitor with an IC50 of 14 μM. 1-Naphthohydroxamic acid is more selectively for HDAC8 than class I HDAC1 and class II HDAC6 (IC50 >100 μM). 1-Naphthohydroxamic acid does not increase global histone H4 acetylation and also does not reduce total intracellular HDAC activity[1][2].1-Naphthohydroxamic acid can induce tubulin acetylation[3]. |
|---|---|
| Related Catalog | |
| Target |
HDAC8:14 μM (IC50) HDAC1:>100 μM (IC50) HDAC6:>100 μM (IC50) |
| In Vitro | 1-Naphthohydroxamic acid (compound 2; 20-40 µM; 0-144 hours; BE(2)-C, SK-N-BE(2) and SH-SY5Y cells) treatment reduces cell numbers in a concentration-dependent manner[2]. 1-Naphthohydroxamic acid (compound 2) at concentrations in the range of its in vitro IC50 against HDAC8 results in reduced cell density and outgrowth of neurite-like structures that stained positive for neurofilament.1-Naphthohydroxamic acid reduces the formation of clones in soft-agar concentration dependently[2]. When either cell type (HeLa and HEK293 cells) is treated with 1-Naphthohydroxamic acid (compound 2; 0.8 µM, 4 µM, 20 µM or 100 µM), only tubulin becomes hyperacetylated[1]. Cell Proliferation Assay[2] Cell Line: BE(2)-C, SK-N-BE(2) and SH-SY5Y cells Concentration: 20 µM, 40 µM Incubation Time: 0 hours, 24 hours, 48 hours, 72 hours, 96 hours, and 144 hours Result: Reduced cell numbers in a concentration-dependent manner. |
| In Vivo | Dose-limiting toxicities (DLTs) of 1-Naphthohydroxamic acid (compound 2; 0-40 mg/kg; intraperitoneal injection; daily; for 10 day; NMRI Foxn1 nude mice) include weight loss and signs of liver toxicity, as evidenced by elevated plasma liver enzymes and detection of necrotic areas on histological liver examination. 1-Naphthohydroxamic acid has the maximum tolerable doses at 50 mg/kg per day. At these concentrations, neither body weight nor blood parameters are critically changed[3]. Pharmacokinetic studies after intraperitoneal administration of the inhibitors identified the half-life of 1-Naphthohydroxamic acid to be ~15 min, with a plasma peak concentration of ~30 μM[3]. Animal Model: NMRI Foxn1 nude mice[3] Dosage: 0 mg/kg, 50 mg/kg, 100mg/kg, 200 mg/kg, 300 mg/kg 400 mg/kg Administration: Intraperitoneal injection; daily; for 10 days Result: Dose-limiting toxicities (DLTs) included weight loss and signs of liver toxicity, as evidenced by elevated plasma liver enzymes and detection of necrotic areas on histological liver examination. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C11H9NO2 |
| Molecular Weight | 187.195 |
| Exact Mass | 187.063324 |
| PSA | 49.33000 |
| LogP | 1.49 |
| Appearance of Characters | white to tan |
| Index of Refraction | 1.678 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: ≥5mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Risk Phrases | 52 |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | QJ1894500 |
| HS Code | 2924299090 |
|
~99% 
1-Naphthohydrox... CAS#:6953-61-3 |
| Literature: Gao, Xi-Ai; Wang, Xian-Xue; Yan, Hao; Li, Jian; Yan, Ru-Long; Huang, Guo-Sheng Journal of the Indian Chemical Society, 2013 , vol. 90, # 3 p. 381 - 385 |
|
~% 
1-Naphthohydrox... CAS#:6953-61-3 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 17, # 10 p. 2874 - 2878 |
|
~% 
1-Naphthohydrox... CAS#:6953-61-3 |
| Literature: Organic Letters, , vol. 8, # 8 p. 1729 - 1732 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| 1-NAPHTHALENECARBOXAMIDE,N-HYDROXY |
| a-Naphthohydroxamic acid |
| N-hydroxynaphthalene-1-carboxamide |
| 1-Naphthalenecarboxamide, N-hydroxy- |
| N-Hydroxy-1-naphthamide |
| 1-naphthohydroxamic acid |
 CAS#:134-32-7
CAS#:134-32-7 CAS#:607-56-7
CAS#:607-56-7