Elvitegravir(GS-9137,JTK-303)
Modify Date: 2024-01-02 17:28:38
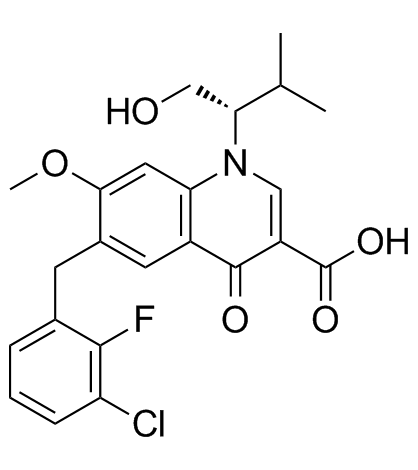
Elvitegravir(GS-9137,JTK-303) structure
|
Common Name | Elvitegravir(GS-9137,JTK-303) | ||
|---|---|---|---|---|
| CAS Number | 697761-98-1 | Molecular Weight | 447.884 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 623.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C23H23ClFNO5 | Melting Point | 93-96°C | |
| MSDS | N/A | Flash Point | 330.9±31.5 °C | |
Use of Elvitegravir(GS-9137,JTK-303)Elvitegravir is an HIV integrase inhibitor for HIV-1IIIB, HIV-2EHO and HIV-2ROD with IC50 of 0.7 nM, 2.8 nM and 1.4 nM, respectively. |
| Name | elvitegravir |
|---|---|
| Synonym | More Synonyms |
| Description | Elvitegravir is an HIV integrase inhibitor for HIV-1IIIB, HIV-2EHO and HIV-2ROD with IC50 of 0.7 nM, 2.8 nM and 1.4 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.7 nM (HIV-1IIIB), 2.8 nM (HIV-2EHO), 1.4 nM(HIV-2ROD)[1] |
| In Vitro | Elvitegravir (EVG) blocks the integration of HIV-1 cDNA through the inhibition of DNA strand transfer. Elvitegravir exerts potent anti-HIV activity against not only wild-type strains but also drug-resistant clinical isolates. Interestingly, Elvitegravir also shows antiviral activity against murine leukemia virus (MLV) and simian immunodeficiency virus (SIV). Elvitegravir shows potent antiviral activity against three laboratory strains of HIV, with EC50 values in the subnanomolar to nanomolar range. Next, the activity of Elvitegravir is evaluated against wild-type clinical isolates representing various subtypes of HIV-1. Elvitegravir suppresses the replication of all HIV-1 subtypes tested, with an antiviral EC50 ranging from 0.1 to 1.26 nM. Moreover, Elvitegravir suppresses the replication of HIV-1 clinical isolates carrying NRTI, NNRTI, and PI resistance-associated genotypes, as did a control IN inhibitor, the compound L-870,810. The cytotoxicities of these inhibitors are also determined using an MTT colorimetric assay. Mean values for the concentration that suppresses the viability of target cells by 50% for Elvitegravir and L-870,810 in PBMC obtained from three independent donors are 4.6±0.5 μM and 2.7±0.6 μM, respectively. Thus, Elvitegravir can suppress various HIV strains, including diverse HIV-1 subtypes and clinical isolates carrying multiple mutations associated with resistance to currently approved antiretroviral drugs[1]. |
| Cell Assay | MT-2 cells (2×105 cells) are infected with HIV-1 IIIB and then cultured in the presence of 0.5 nM or 0.1 nM Elvitegravir. Cultures are incubated at 37°C until an extensive cytopathic effect (CPE) is observed, and the culture supernatant is then harvested for further passage in fresh MT-2 cells. The concentration of Elvitegravir is increased when a significant CPE is observed. At the indicated passages, proviral DNA is extracted from infected MT-2 cells and then subjected to PCR, followed by direct population-based sequencing. Susceptibility to Elvitegravir at the indicated passages is determined using the MAGI assay or p24 production[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 623.6±55.0 °C at 760 mmHg |
| Melting Point | 93-96°C |
| Molecular Formula | C23H23ClFNO5 |
| Molecular Weight | 447.884 |
| Flash Point | 330.9±31.5 °C |
| Exact Mass | 447.124878 |
| PSA | 88.76000 |
| LogP | 4.29 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.603 |
| Storage condition | Refrigerator |
| 3-Quinolinecarboxylic acid, 6-[(3-chloro-2-fluorophenyl)methyl]-1,4-dihydro-1-[(1S)-1-(hydroxymethyl)-2-methylpropyl]-7-methoxy-4-oxo- |
| 6-(3-Chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methyl-2-butanyl]-7-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid |
| UNII-4GDQ854U53 |
| 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid |
| Elvitegravir |
| MFCD11846134 |
| GS9137 |