H-Phe-Gly-OH

H-Phe-Gly-OH structure
|
Common Name | H-Phe-Gly-OH | ||
|---|---|---|---|---|
| CAS Number | 721-90-4 | Molecular Weight | 222.24000 | |
| Density | 1.259g/cm3 | Boiling Point | 512.5ºC at 760 mmHg | |
| Molecular Formula | C11H14N2O3 | Melting Point | -260ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 263.7ºC | |
Use of H-Phe-Gly-OHPhe-Gly hydrate is a Glycine (HY-Y0966) derivative[1]. |
| Name | h-phe-gly-oh |
|---|---|
| Synonym | More Synonyms |
| Description | Phe-Gly hydrate is a Glycine (HY-Y0966) derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.259g/cm3 |
|---|---|
| Boiling Point | 512.5ºC at 760 mmHg |
| Melting Point | -260ºC (dec.) |
| Molecular Formula | C11H14N2O3 |
| Molecular Weight | 222.24000 |
| Flash Point | 263.7ºC |
| Exact Mass | 222.10000 |
| PSA | 92.42000 |
| LogP | 0.84840 |
| Index of Refraction | 1.576 |
| Storage condition | -20C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2924299090 |
| Precursor 7 | |
|---|---|
| DownStream 4 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Hydrogen exchange equilibria in thiols.
Chem. Res. Toxicol. 25(9) , 1862-7, (2012) Cysteine, cysteinyl-glycine, glutathione, phenylalanyl-cysteinyl-glycine, and histidyl-cysteinyl-glycine were dissolved in acidic and neutral D(2)O in the presence of the radical generator 2,2'-azobis... |
|
|
Enhanced transdermal delivery of phenylalanyl-glycine by chemical modification with various fatty acids.
Int. J. Pharm. 250(1) , 119-28, (2003) We synthesized three novel lipophilic derivatives of phenylalanyl-glycine (Phe-Gly), C4-Phe-Gly, C6-Phe-Gly and C8-Phe-Gly by chemical modification with butyric acid (C4), caproic acid (C6) and octano... |
|
|
Enhancement of the small intestinal uptake of phenylalanylglycine via a H+/oligopeptide transport system by chemical modification with fatty acids.
Life Sci. 61(25) , 2455-65, (1997) The transport characteristics of chemically modified phenylalanylglycine (Phe-Gly) with butyric acid (C4-Phe-Gly) and caproic acid (C6-Phe-Gly) were examined using rabbit intestinal brush-border membr... |
| Phe-Gly-OH |
| Phe-glycrystalline |
| L-Phenylalanyl-glycin |
| phenylalanylglycine |
| Phe-Gly hydrate |
| L-Phe-Gly-OH |
| L-PHENYLALANYLGLYCINE |
| phenylalanyl-glycin |
| phenylalanineglycine |
| PHE-GLY |
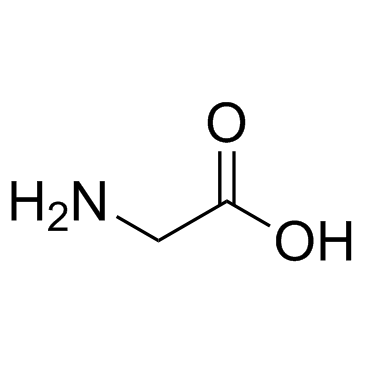 CAS#:56-40-6
CAS#:56-40-6 CAS#:583-47-1
CAS#:583-47-1 CAS#:63-91-2
CAS#:63-91-2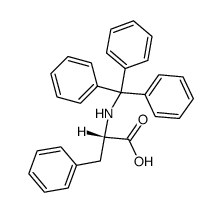 CAS#:47672-25-3
CAS#:47672-25-3 CAS#:2577-90-4
CAS#:2577-90-4![L-Phenylalanine,N-[(2-nitrophenyl)thio]- Structure](https://image.chemsrc.com/caspic/176/2688-22-4.png) CAS#:2688-22-4
CAS#:2688-22-4![N-[2-Nitro-benzolsulfenyl]-L-Phe-Gly-OEt Structure](https://image.chemsrc.com/caspic/448/7754-66-7.png) CAS#:7754-66-7
CAS#:7754-66-7 CAS#:169624-67-3
CAS#:169624-67-3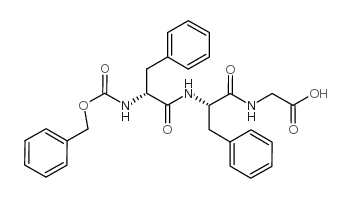 CAS#:75539-79-6
CAS#:75539-79-6
