swainsonine
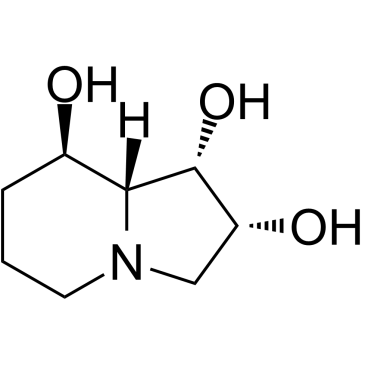
swainsonine structure
|
Common Name | swainsonine | ||
|---|---|---|---|---|
| CAS Number | 72741-87-8 | Molecular Weight | 173.210 | |
| Density | 1.38±0.1 g/cm3 | Boiling Point | 353.3±21.0 °C at 760 mmHg | |
| Molecular Formula | C8H15NO3 | Melting Point | 144-145 ºC | |
| MSDS | Chinese USA | Flash Point | 209.7±20.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of swainsonineSwainsonine is an alkaloid isolated from Astragalus, acts as an inhibitor of α-mannosidase, with anti-tumor activity[1]. |
| Name | swainsonine |
|---|---|
| Synonym | More Synonyms |
| Description | Swainsonine is an alkaloid isolated from Astragalus, acts as an inhibitor of α-mannosidase, with anti-tumor activity[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.38±0.1 g/cm3 |
|---|---|
| Boiling Point | 353.3±21.0 °C at 760 mmHg |
| Melting Point | 144-145 ºC |
| Molecular Formula | C8H15NO3 |
| Molecular Weight | 173.210 |
| Flash Point | 209.7±20.7 °C |
| Exact Mass | 173.105194 |
| PSA | 63.93000 |
| LogP | -0.79 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.609 |
| Storage condition | 2-8°C |
| Water Solubility | Freely soluble (170 g/L) (25 ºC) |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H332 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | 36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NM2408666 |
| HS Code | 29339900 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
|
Production, HPLC analysis, and in situ apoptotic activities of swainsonine toward lepidopteran, Sf-21 cell line.
Biotechnol. Prog. 30(5) , 1196-205, (2014) Swainsonine, a secondary metabolite from Metarhizium anisopliae has been extensively studied in the complementary areas of therapeutics and toxicology. This work aims to develop a simple UV-HPLC metho... |
|
|
DrugBank 3.0: a comprehensive resource for 'omics' research on drugs.
Nucleic Acids Res. 39 , D1035-41., (2011) DrugBank (http://www.drugbank.ca) is a richly annotated database of drug and drug target information. It contains extensive data on the nomenclature, ontology, chemistry, structure, function, action, ... |
|
|
Swainsonine inhibits growth and potentiates the cytotoxic effect of paclitaxel in hepatocellular carcinoma in vitro and in vivo.
Oncol. Rep. 28(6) , 2091-100, (2012) Swainsonine, an extract from Astragalus membranaceus, exhibits broad inhibition of growth and pro-apoptotic activity in a number of tumor types. However, the underlying mechanism involved remains uncl... |
| D-Swainsonine |
| (1S,2R,8R,8aR)-Octahydroindolizine-1,2,8-triol |
| (1S,2R,8R,8aR)-Octahydro-1,2,8-indolizinetriol |
| 1,2,8-Indolizinetriol, octahydro-, (1S,2R,8R,8aR)- |
| 8A,B-INDOLIZIDINE-1,2A,8B-TRIOL |
| Tridolgosir |
| SwainMoia |
| UNII-RSY4RK37KQ |
| swainsonine synthetic |
| Swainsonine |
| MFCD00017554 |
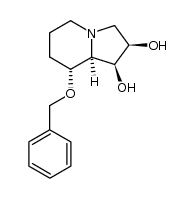 CAS#:162470-36-2
CAS#:162470-36-2 CAS#:85624-09-5
CAS#:85624-09-5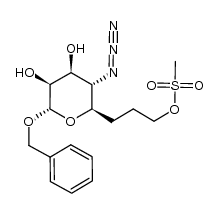 CAS#:1002754-00-8
CAS#:1002754-00-8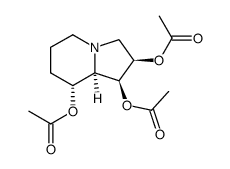 CAS#:72741-89-0
CAS#:72741-89-0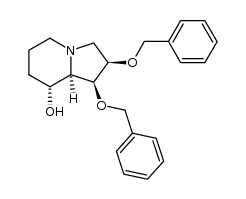 CAS#:130412-86-1
CAS#:130412-86-1 CAS#:129421-04-1
CAS#:129421-04-1![[1S,2R,8R,8aR]-8-Triisopropylsilyloxy-1,2-(isopropylidenedioxy)indolizidine Structure](https://image.chemsrc.com/caspic/047/253778-10-8.png) CAS#:253778-10-8
CAS#:253778-10-8![(3aR,9R,9aS,9bS)-9-(tert-butyldimethylsilyloxy)-2,2-dimethyl-octahydro-[1,3]dioxolo[4,5-a]indolizine Structure](https://image.chemsrc.com/caspic/372/1148122-99-9.png) CAS#:1148122-99-9
CAS#:1148122-99-9![(3aR,9R,9aR,9bS)-octahydro-2,2-dimethyl-9-(phenylmethoxy)-1,3-dioxolo[4,5-a]indolizine Structure](https://image.chemsrc.com/caspic/449/154815-12-0.png) CAS#:154815-12-0
CAS#:154815-12-0
