E-64c
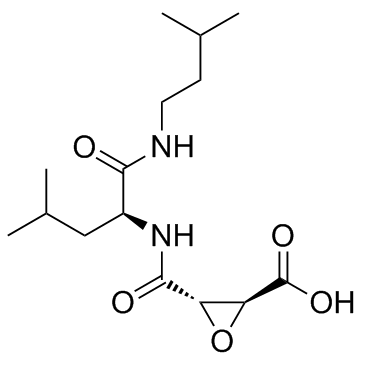
E-64c structure
|
Common Name | E-64c | ||
|---|---|---|---|---|
| CAS Number | 76684-89-4 | Molecular Weight | 314.377 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 596.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C15H26N2O5 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 314.5±30.1 °C | |
Use of E-64cE 64c is a derivative of naturally occurring epoxide inhibitor of cysteine proteases, a Calcium-activated neutral protease (CANP) inhibitor and a very weak irreversible cathepsin C inhibitor. |
| Name | e-64c |
|---|---|
| Synonym | More Synonyms |
| Description | E 64c is a derivative of naturally occurring epoxide inhibitor of cysteine proteases, a Calcium-activated neutral protease (CANP) inhibitor and a very weak irreversible cathepsin C inhibitor. |
|---|---|
| Related Catalog | |
| Target |
Cysteine proteases[1], CANP[2], Cathepsin C[3]. |
| In Vitro | E-64c, a derivative of naturally occurring epoxide inhibitor of cysteine proteases, with papain; especially with regard to the hydrogen bonding and hydrophobic interactions of the ligands with conserved residues in the catalytic binding site[1]. E 64c (k2/Ki=140±5M-1s-1) is demonstrated to be a lead structure for the development of irreversible cathepsin C inhibitors[3]. |
| In Vivo | The t-1/2 of plasma E-64c is 0.48 hours. The hemodynamic effects of E-64c are absent at this dose. Using two way analysis of variance, the effects of reperfusion (p=0.0016) or E-64c (p=0.0226) per se on infarct size are significant. In comparing Group A with Group B and Group C with Group D, the depletion of CPK in the E-64c treated groups (Groups A and C) is slightly less than in the vehicle-injected groups (Groups B and D). The insufficient effect of E-64c alone may be explained by the early administration and relatively short t-1/2. Since the effectiveness of NCO-700 has been established,6),7) our findings might indicate a small but beneficial effect of E-64c on infarct size and CPK content[2]. |
| Animal Admin | Dogs[2] Studies are carried out in 83 mongrel dogs with a mean weight of 11.2kg. They are anesthetized with intravenous sodium thiamylal (7mg/kg). An intravenous bolus of E-64c (100mg/kg), dissolved in saturated sodium bicarbonate, is administered immediately before the occlusion and after reperfusion in Group A (n=17), whereas Group B (n=17) receive only the vehicle solution at these times. In the remaining 49 dogs (Groups C and D), the LAD is permanently ligated at the same level and an intravenous bolus of either Loxistatin acid (100mg/kg) (Group C; n=24) or vehicle only (Group D; n=25) is given immediately before and 1 hour after the ligation. The dose of E-64c is designed for its possible use in clinical practice and the estimated intramyocardial Loxistatin acid molecular concentration is 1,000 times that of total mCANP[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 596.4±50.0 °C at 760 mmHg |
| Molecular Formula | C15H26N2O5 |
| Molecular Weight | 314.377 |
| Flash Point | 314.5±30.1 °C |
| Exact Mass | 314.184174 |
| PSA | 108.03000 |
| LogP | 1.36 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.504 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | RR0404200 |
|
Nitro/nitrosyl-ruthenium complexes are potent and selective anti-Trypanosoma cruzi agents causing autophagy and necrotic parasite death.
Antimicrob. Agents Chemother. 58(10) , 6044-55, (2014) cis-[RuCl(NO2)(dppb)(5,5'-mebipy)] (complex 1), cis-[Ru(NO2)2(dppb)(5,5'-mebipy)] (complex 2), ct-[RuCl(NO)(dppb)(5,5'-mebipy)](PF6)2 (complex 3), and cc-[RuCl(NO)(dppb)(5,5'-mebipy)](PF6)2 (complex 4... |
|
|
HNA antibody-mediated neutrophil aggregation is dependent on serine protease activity.
Vox Sang. 109 , 366-74, (2015) Transfusion-related acute lung injury (TRALI) is often caused by antibodies against human neutrophil alloantigen-2 (HNA-2) and HNA-3a. Neutrophil aggregation is considered as a major cause of TRALI, b... |
|
|
Structural basis for development of cathepsin B-specific noncovalent-type inhibitor: crystal structure of cathepsin B-E64c complex.
Biochim. Biophys. Acta 1597(2) , 244-51, (2002) In order to elucidate the substrate specificity of the Sn subsites (n=1-3) of cathepsin B, its crystal structure inhibited by E64c [(+)-(2S,3S)-3-(1-[N-(3-methylbutyl)amino]-leucylcarbonyl)oxirane-2-c... |
| 2-Oxiranecarboxylic acid, 3-[[[(1S)-3-methyl-1-[[(3-methylbutyl)amino]carbonyl]butyl]amino]carbonyl]-, (2S,3S)- |
| Loxistatin acid |
| (2S,3S)-3-({(2S)-4-Methyl-1-[(3-methylbutyl)amino]-1-oxo-2-pentanyl}carbamoyl)-2-oxiranecarboxylic acid |
| (2S,3S)-3-({(2S)-4-methyl-1-[(3-methylbutyl)amino]-1-oxopentan-2-yl}carbamoyl)oxirane-2-carboxylic acid |
| L-TRANS-EPOXYSUCCINYL-LEU-3-METHYLBUTYLAMIDE |
| E-64c,EP 475 |
| Inhibitorforthiolprotease |
| anecarboxylicacid |

