cis-Urocanic acid
Modify Date: 2024-01-13 12:17:14
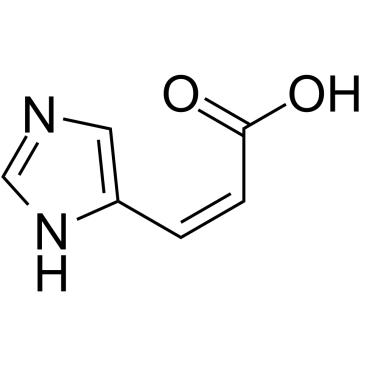
cis-Urocanic acid structure
|
Common Name | cis-Urocanic acid | ||
|---|---|---|---|---|
| CAS Number | 7699-35-6 | Molecular Weight | 138.12400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H6N2O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of cis-Urocanic acidcis-Urocanic acid is a 5-HT2A receptor agonist. cis-Urocanic acid binds to 5-HT receptor with relatively high affinity (Kd=4.6 nM). cis-Urocanic acid is an immune modulator that induces immunosuppression by binding to the 5-HT2A receptor[1]. |
| Name | cis-urocanic acid |
|---|---|
| Synonym | More Synonyms |
| Description | cis-Urocanic acid is a 5-HT2A receptor agonist. cis-Urocanic acid binds to 5-HT receptor with relatively high affinity (Kd=4.6 nM). cis-Urocanic acid is an immune modulator that induces immunosuppression by binding to the 5-HT2A receptor[1]. |
|---|---|
| Related Catalog | |
| Target |
5-HT2A Receptor |
| In Vitro | Treatment with 100 μg/mL cis-Urocanic acid (cis-UCA) completely suppresses IL-6 and IL-8 secretion, decreases caspase-3 activity, and improves cell viability against UV-B irradiation. No significant effects on IL-6 or IL-8 secretion, caspase-3 activity, or viability of the non-irradiated cells are observed with 100 μg/mL cis-Urocanic acid in both cell types. The 5000 μg/mL concentration is toxic[1]. Cell Viability Assay[1] Cell Line: Human corneal epithelial cells (HCE-2) and human conjunctival epithelial cells (HCECs) Concentration: 10, 100, 1,000, and 5,000 μg/mL Incubation Time: 24, 48, or 72 hours Result: Treatment with 100 μg/mL completely suppressed IL-6 and IL-8 secretion, decreased caspase-3 activity, and improved cell viability against UV-B irradiation. No significant effects on IL-6 or IL-8 secretion, caspase-3 activity, or viability of the non-irradiated cells were observed with 100 μg/mL in both cell types. |
| References |
| Molecular Formula | C6H6N2O2 |
|---|---|
| Molecular Weight | 138.12400 |
| Exact Mass | 138.04300 |
| PSA | 65.98000 |
| LogP | 0.50750 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933290090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Investigation of a Degradant in a Biologics Formulation Buffer Containing L-Histidine.
Pharm. Res. 32 , 2625-35, (2015) An unknown UV 280 nm absorbing peak was observed by SEC for protein stability samples formulated in L-histidine during a stress stability study. Understanding the source would enhance the confidence i... |
| 3-[1H-imidazol-4(5)-yl] |
| Cis-Urocanic Acid-[13C3] |
| (E)-3-(3H-imidazol-4-yl)prop-2-enoic acid |
| (Z)-Urocanic acid |
| cis-Urocanic acid |
| (Z)-2-propenoic acid |
| (Z)-3-(1H-imidazol-4-yl)-2-propenoic acid |
| (2Z)-3-(1H-Imidazole-4-yl)acrylic acid |
| (Z)-3-(1H-Imidazole-4-yl)acrylic acid |
| (Z)-3-(1H-imidazol-4-yl)prop-2-enoic acid |
| UROCANIC ACID, CIS |
| (Z)-3-(1H-Imidazol-4-yl)propenoic acid |
 CAS#:3465-72-3
CAS#:3465-72-3