UNII:3I7LZ8M32B
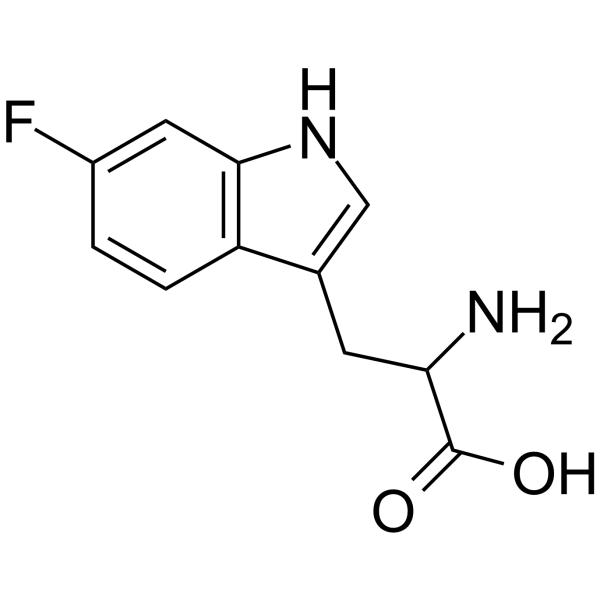
UNII:3I7LZ8M32B structure
|
Common Name | UNII:3I7LZ8M32B | ||
|---|---|---|---|---|
| CAS Number | 7730-20-3 | Molecular Weight | 222.216 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 450.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C11H11FN2O2 | Melting Point | 280-285 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 226.4±28.7 °C | |
Use of UNII:3I7LZ8M32B6-Fluorotryptophan, a competitive inhibitor of tryptophan hydroxylase, produced a transient disruption of sleep in rats chronically implanted with EEG recording electrodes[1]. |
| Name | 6-Fluoro-DL-tryptophan |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Fluorotryptophan, a competitive inhibitor of tryptophan hydroxylase, produced a transient disruption of sleep in rats chronically implanted with EEG recording electrodes[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 450.7±45.0 °C at 760 mmHg |
| Melting Point | 280-285 °C (dec.)(lit.) |
| Molecular Formula | C11H11FN2O2 |
| Molecular Weight | 222.216 |
| Flash Point | 226.4±28.7 °C |
| Exact Mass | 222.080460 |
| PSA | 79.11000 |
| LogP | 1.09 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.673 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Substrate promiscuity of the cyclic dipeptide prenyltransferases from Aspergillus fumigatus ( section sign).
J. Nat. Prod. 72 , 44-52, (2009) This study reports that a series of tryptophan derivatives with modifications on the side chain or at the indole ring were accepted by two cyclic dipeptide prenyltransferases, CdpNPT and FtmPT1, and c... |
|
|
Cloning of the trp gene cluster from a tryptophan-hyperproducing strain of Corynebacterium glutamicum: identification of a mutation in the trp leader sequence.
Appl. Environ. Microbiol. 59(3) , 791-9, (1993) Corynebacterium glutamicum ATCC 21850 produces up to 5 g of extracellular L-tryptophan per liter in broth culture and displays resistance to several synthetic analogs of aromatic amino acids. Here we ... |
|
|
Atomic mutations at the single tryptophan residue of human recombinant annexin V: effects on structure, stability, and activity.
Biochemistry 38(33) , 10649-59, (1999) The single tryptophan residue (Trp187) of human recombinant annexin V, containing 320 residues and 5328 atoms, was replaced with three different isosteric analogues where hydrogen atoms at positions 4... |
| EINECS 231-788-6 |
| Tryptophan, 6-fluoro- |
| 2-Amino-3-(6-fluoro-1H-indol-3-yl)propanoic acid |
| 6-Fluorotryptophan |
| 6-fluoro-dl-tryptophan |
| MFCD00005653 |
| UNII:3I7LZ8M32B |
![methyl 2-(9H-pyrido[3,4-b]indol-1-yl)acetate structure](https://image.chemsrc.com/caspic/302/138428-34-9.png) CAS#:138428-34-9
CAS#:138428-34-9
