Loteprednol etabonate
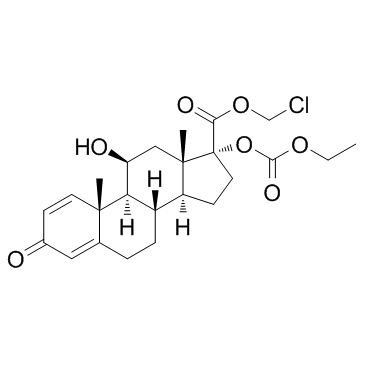
Loteprednol etabonate structure
|
Common Name | Loteprednol etabonate | ||
|---|---|---|---|---|
| CAS Number | 82034-46-6 | Molecular Weight | 466.952 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 600.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C24H31ClO7 | Melting Point | 220.5-223.5ºC | |
| MSDS | Chinese USA | Flash Point | 316.7±31.5 °C | |
Use of Loteprednol etabonateLoteprednol Etabonate is an anti-inflammatory corticosteroid used in optometry and ophthalmology.IC50 Value:Target: Glucocorticoid Receptorin vitro:in vivo: Intravenous administration of loteprednol etabonate (5 mg/kg) to dogs revealed a terminal half-life of 2.8 h, a volume of distribution of 3.7 L/kg, and a total body clearance of 0.9 L/h/kg. Intact loteprednol etabonate was not detectable in the urine. After oral administration of the drug (5 mg/kg) to dogs, only metabolites, but no intact drug, were found in the plasma, an indication for a high first-pass effect. A pronounced binding of the drug to plasma protein (> 90%) and a high erythrocyte-buffer partition coefficient of 7.8 were determined in vitro. |
| Name | loteprednol etabonate |
|---|---|
| Synonym | More Synonyms |
| Description | Loteprednol Etabonate is an anti-inflammatory corticosteroid used in optometry and ophthalmology.IC50 Value:Target: Glucocorticoid Receptorin vitro:in vivo: Intravenous administration of loteprednol etabonate (5 mg/kg) to dogs revealed a terminal half-life of 2.8 h, a volume of distribution of 3.7 L/kg, and a total body clearance of 0.9 L/h/kg. Intact loteprednol etabonate was not detectable in the urine. After oral administration of the drug (5 mg/kg) to dogs, only metabolites, but no intact drug, were found in the plasma, an indication for a high first-pass effect. A pronounced binding of the drug to plasma protein (> 90%) and a high erythrocyte-buffer partition coefficient of 7.8 were determined in vitro. |
|---|---|
| Related Catalog | |
| References |
[6]. Loteprednol |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 600.1±55.0 °C at 760 mmHg |
| Melting Point | 220.5-223.5ºC |
| Molecular Formula | C24H31ClO7 |
| Molecular Weight | 466.952 |
| Flash Point | 316.7±31.5 °C |
| Exact Mass | 466.175842 |
| PSA | 99.13000 |
| LogP | 3.17 |
| Appearance of Characters | white to beige |
| Vapour Pressure | 0.0±3.9 mmHg at 25°C |
| Index of Refraction | 1.571 |
| Storage condition | -20°C Freezer |
| Water Solubility | DMSO: soluble5mg/mL, clear (warmed) |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2937229000 |
| HS Code | 2937229000 |
|---|
|
Topical cyclosporine a 1% for the treatment of chronic ocular surface inflammation.
Eye Contact Lens 40(5) , 283-8, (2014) To evaluate the use of topical cyclosporine A (CsA) 1% emulsion in the treatment of chronic ocular surface inflammation (OSI).We conducted a retrospective chart review of patients with various forms o... |
|
|
Small-incision lenticule extraction for myopia: results of a 12-month prospective study.
Optom. Vis. Sci. 92(1) , 123-31, (2015) To analyze the safety, efficacy, stability, and predictability of small-incision lenticule extraction to correct myopia.Patients were evaluated preoperatively and then at 1 day, at 2 weeks, and at 1, ... |
|
|
Loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgery.
J. Cataract Refract. Surg. 39(2) , 168-73, (2013) To evaluate the efficacy of loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the control of postoperative inflammation in patients having routine cataract surgery.Private practice, Stil... |
| Androsta-1,4-diene-17-carboxylic acid, 17-((ethoxycarbonyl)oxy)-11-hydroxy-3-oxo-, chloromethyl ester, (11β,17α)- |
| chloromethyl (8S,9S,10R,11S,13S,14S,17R)-17-[(ethoxycarbonyl)oxy]-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthrene-17-carboxylate |
| (8S,9S,10R,11S,13S,14S,17R)-17-[(éthoxycarbonyl)oxy]-11-hydroxy-10,13-diméthyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodécahydro-3H-cyclopenta[a]phénanthrène-17-carboxylate de chlorométhyle |
| Chloromethyl 17a-ethoxycarbonyloxy-11b-hydroxyandrosta-1,4-diene-3-one-17b-carboxylate |
| (11b,17a)-17-[(Ethoxycarbonyl)oxy]-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylic Acid Chloromethyl Ester |
| Loteprednol (INN) |
| Androsta-1,4-diene-17-carboxylic acid, 17-[(ethoxycarbonyl)oxy]-11-hydroxy-3-oxo-, chloromethyl ester, (11β,17α)- |
| Loteprednolum |
| Loteprednol Etabonate |
| 17a-Ethoxycarbonyloxy-D'-cortienic Acid Chloromethyl Ester |
| Loteprednol [INN:BAN] |
| Chloromethyl (11β,17α)-17-[(ethoxycarbonyl)oxy]-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylate |
| HGP 1 |
| loteprednol |
| Chlormethyl-(8S,9S,10R,11S,13S,14S,17R)-17-[(ethoxycarbonyl)oxy]-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-carboxylat |