2-Chlorophenothiazine
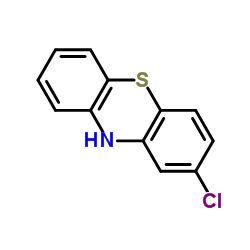
2-Chlorophenothiazine structure
|
Common Name | 2-Chlorophenothiazine | ||
|---|---|---|---|---|
| CAS Number | 92-39-7 | Molecular Weight | 233.717 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 395.7±31.0 °C at 760 mmHg | |
| Molecular Formula | C12H8ClNS | Melting Point | 195-200 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 193.1±24.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2-Chlorophenothiazine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 395.7±31.0 °C at 760 mmHg |
| Melting Point | 195-200 °C(lit.) |
| Molecular Formula | C12H8ClNS |
| Molecular Weight | 233.717 |
| Flash Point | 193.1±24.8 °C |
| Exact Mass | 233.006592 |
| PSA | 37.33000 |
| LogP | 5.18 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.680 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | SN7580000 |
| Hazard Class | 6.1 |
| HS Code | 2934300000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934300000 |
|---|---|
| Summary | 2934300000. other compounds containing in the structure a phenothiazine ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Reversing the Warburg effect as a treatment for glioblastoma.
J. Biol. Chem. 288(13) , 9153-64, (2013) Glioblastoma multiforme (GBM), like most cancers, possesses a unique bioenergetic state of aerobic glycolysis known as the Warburg effect. Here, we documented that methylene blue (MB) reverses the War... |
|
|
Broad specific enzyme-linked immunosorbent assay for determination of residual phenothiazine drugs in swine tissues.
Anal. Biochem. 454 , 7-13, (2014) In this study, a novel generic hapten of phenothiazine drugs was synthesized by derivatization of 2-chlorophenothiazine with sodium bromoacetate. Then the hapten was coupled to bovine serum albumin fo... |
|
|
N-benzoylated phenoxazines and phenothiazines: synthesis, antiproliferative activity, and inhibition of tubulin polymerization.
J. Med. Chem. 54 , 4247-63, (2011) A total of 53 N-benzoylated phenoxazines and phenothiazines, including their S-oxidized analogues, were synthesized and evaluated for antiproliferative activity, interaction with tubulin, and cell cyc... |
| EINECS 202-152-5 |
| 10H-Phenothiazine, 2-chloro- |
| 2-Chloro-10H-phenothiazine |
| MFCD00005016 |
 CAS#:583-55-1
CAS#:583-55-1 CAS#:1004-00-8
CAS#:1004-00-8 CAS#:108-94-1
CAS#:108-94-1 CAS#:694-80-4
CAS#:694-80-4 CAS#:583-53-9
CAS#:583-53-9 CAS#:615-41-8
CAS#:615-41-8 CAS#:105790-02-1
CAS#:105790-02-1 CAS#:101-17-7
CAS#:101-17-7![acetic acid-[2-(5-chloro-2-nitro-phenylsulfanyl)-anilide] Structure](https://image.chemsrc.com/caspic/127/107522-19-0.png) CAS#:107522-19-0
CAS#:107522-19-0 CAS#:5182-81-0
CAS#:5182-81-0![10-[2-methyl-3-(methylamino)propyl]phenothiazine-2-carbonitrile structure](https://image.chemsrc.com/caspic/205/108014-19-3.png) CAS#:108014-19-3
CAS#:108014-19-3 CAS#:38642-74-9
CAS#:38642-74-9 CAS#:3546-03-0
CAS#:3546-03-0 CAS#:50-53-3
CAS#:50-53-3 CAS#:19607-03-5
CAS#:19607-03-5 CAS#:63615-79-2
CAS#:63615-79-2 CAS#:1927-43-1
CAS#:1927-43-1 CAS#:92-84-2
CAS#:92-84-2 CAS#:2095-17-2
CAS#:2095-17-2 CAS#:2002-32-6
CAS#:2002-32-6
