SB743921
Modify Date: 2024-01-02 19:46:25
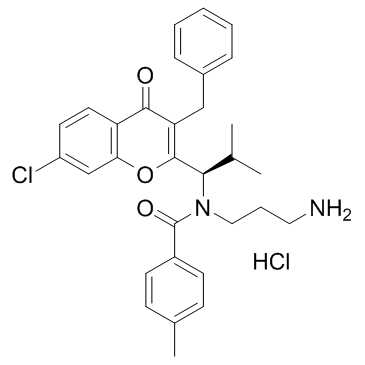
SB743921 structure
|
Common Name | SB743921 | ||
|---|---|---|---|---|
| CAS Number | 940929-33-9 | Molecular Weight | 553.519 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C31H34Cl2N2O3 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of SB743921SB-743921 is a potent inhibitor of the mitotic kinesin KSP (Eg5), with a Ki of 0.1 nM. |
| Name | Benzamide, N-(3-aminopropyl)-N-[(1R)-1-[7-chloro-4-oxo-3-(phenylmethyl)-4H-1-benzopyran-2-yl]-2-methylpropyl]-4-methyl-, hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | SB-743921 is a potent inhibitor of the mitotic kinesin KSP (Eg5), with a Ki of 0.1 nM. |
|---|---|
| Related Catalog | |
| Target |
Eg5:0.1 nM (Ki) |
| In Vitro | SB-743921 is a potent inhibitor of Eg5, with a Ki of 0.1 nM[1]. SB-743921 (1 nM) potently inhibits colony forming cell (CFC) formation of chronic myeloid leukemia (CML) primary cells, but exhibits slight inhibitory activities on the colony-forming ability of normal bone marrow progenitors. SB-743921 (1, 3 nM) induces apoptosis of CML primary CD34 + cells, and shows slight effect on normal CD34 + cells. SB-743921 (2 nM) in combination with imatinib displays additive anti-proliferative effect in KCL22 and CML CD34 + cells. Furthermore, SB-743921 overcomes imatinib resistance in CML cells. SB-743921 (0.5 nM, 1 nM, 3 nM) inhibits MEK/ERK and AKT signaling in CML cells[2]. |
| In Vivo | SB-743921 has good oral bioavailability and pharmacokinetics and induces complete tumor regression in nude mice bearing lung cancer patient xenografts[3]. |
| Cell Assay | K562 and KCL22 cells are seeded in six-well plates at a number of 5 × 105 in 2 mL RPMI-1640 medium supplemented with 10% FBS in a 5% CO2 atmosphere at 37°C, and are treated with control (2% DMSO), 50 nM imatinib, 2 nM SB-743921 and 50 nM imatinib + 2 nM SB-743921, respectively. Cell number and viability are determined every 24 h. Results are plotted for live cells against time to generate a growth curve[2]. |
| Animal Admin | The animal experiments are performed with female NMRI nu/nu mice. Tumor fragments are obtained from xenografts in serial passage in nude mice. Mice are randomized to the various groups, and dosing is started when the required number of mice carries a tumor of 50-250 mm3 volume, preferably 80-200 mm3. Vehicle for 1: 10% ethanol, 10% cremophor, 80% D5W (dextrose 5%); vehicle for all other compounds (including SB-743921): 8% DMSO, 2% Tween 80, distilled water (pH 5). All treatments are given intraperitoneally. Vehicle control mice (group 1) are treated with 10 mL/kg vehicle on days 0, 3, 6, 8, 10, 13, 20, 22, 24, 29, 31, 34, 36, 38, 48, 51, 55, 58, 62, 65, and 69[3]. |
| References |
| Molecular Formula | C31H34Cl2N2O3 |
|---|---|
| Molecular Weight | 553.519 |
| Exact Mass | 552.194641 |
| PSA | 76.54000 |
| LogP | 8.03620 |
| Appearance of Characters | white to beige |
| Storage condition | ?20°C |
| Water Solubility | H2O: soluble5mg/mL, clear |
| RIDADR | NONH for all modes of transport |
|---|
| N-(3-Aminopropyl)-N-[(1R)-1-(3-benzyl-7-chloro-4-oxo-4H-chromen-2-yl)-2-methylpropyl]-4-methylbenzamide hydrochloride (1:1) |
| Benzamide, N-(3-aminopropyl)-N-[(1R)-1-[7-chloro-4-oxo-3-(phenylmethyl)-4H-1-benzopyran-2-yl]-2-methylpropyl]-4-methyl-, hydrochloride (1:1) |
| SB 743921 |
| SB-743921 |