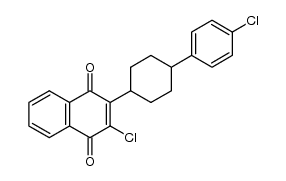
Atovaquone
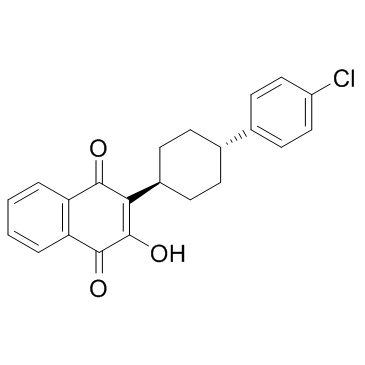
Atovaquone structure
|
Common Name | Atovaquone | ||
|---|---|---|---|---|
| CAS Number | 95233-18-4 | Molecular Weight | 366.837 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 542.2±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H19ClO3 | Melting Point | 216-2190C | |
| MSDS | Chinese USA | Flash Point | 281.7±30.1 °C | |
Use of AtovaquoneAtovaquone is a medication used to treat or prevent for pneumocystis pneumonia, toxoplasmosis, malaria, and babesia.Target: AntiparasiticAtovaquone (atavaquone) is a chemical compound that belongs to the class of naphthalenes. Atovaquone is a hydroxy-1,4-naphthoquinone, an analog of ubiquinone, with antipneumocystic activity [1]. Atovaquone is an anti-protozoal mitochondrial electron transport inhibitor; Antimalarial; Antipneumocystic, and has also been used to treat toxoplasmosis. It acts by inhibiting the cytochrome bc(1) complex via interactions with the Rieske iron-sulfur protein and cytochrome b in the ubiquinol oxidation pocket [2]. Atovaquone is a unique naphthoquinone with broad-spectrum antiprotozoal activity. It is effective for the treatment and prevention of Pneumocystis carinii pneumonia (PCP), it is effective in combination with proguanil for the treatment and prevention of malaria, and it is effective in combination with azithromycin for the treatment of babesiosis [3]. |
| Name | atovaquone |
|---|---|
| Synonym | More Synonyms |
| Description | Atovaquone is a medication used to treat or prevent for pneumocystis pneumonia, toxoplasmosis, malaria, and babesia.Target: AntiparasiticAtovaquone (atavaquone) is a chemical compound that belongs to the class of naphthalenes. Atovaquone is a hydroxy-1,4-naphthoquinone, an analog of ubiquinone, with antipneumocystic activity [1]. Atovaquone is an anti-protozoal mitochondrial electron transport inhibitor; Antimalarial; Antipneumocystic, and has also been used to treat toxoplasmosis. It acts by inhibiting the cytochrome bc(1) complex via interactions with the Rieske iron-sulfur protein and cytochrome b in the ubiquinol oxidation pocket [2]. Atovaquone is a unique naphthoquinone with broad-spectrum antiprotozoal activity. It is effective for the treatment and prevention of Pneumocystis carinii pneumonia (PCP), it is effective in combination with proguanil for the treatment and prevention of malaria, and it is effective in combination with azithromycin for the treatment of babesiosis [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 542.2±50.0 °C at 760 mmHg |
| Melting Point | 216-2190C |
| Molecular Formula | C22H19ClO3 |
| Molecular Weight | 366.837 |
| Flash Point | 281.7±30.1 °C |
| Exact Mass | 366.102264 |
| PSA | 54.37000 |
| LogP | 5.86 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.653 |
| Storage condition | -20°C Freezer |
| Hazard Statements | H413 |
|---|---|
| Hazard Codes | N |
| Risk Phrases | 50/53 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | QJ5616500 |
| HS Code | 2942000000 |
|
~85% 
Atovaquone CAS#:95233-18-4 |
| Literature: WO2010/1378 A1, ; Page/Page column 11 ; |
|
~78% 
Atovaquone CAS#:95233-18-4 |
| Literature: US2010/137644 A1, ; Page/Page column 3-4 ; |
|
~93% 
Atovaquone CAS#:95233-18-4 |
| Literature: Tetrahedron Letters, , vol. 39, # 42 p. 7629 - 7632 |
|
~% 
Atovaquone CAS#:95233-18-4 |
| Literature: WO2012/153162 A1, ; Page/Page column 33 ; |
|
~% 
Atovaquone CAS#:95233-18-4 |
| Literature: Tetrahedron Letters, , vol. 39, # 42 p. 7629 - 7632 |
|
~74% 
Atovaquone CAS#:95233-18-4 |
| Literature: WO2013/14486 A1, ; Page/Page column 27; 28 ; |
|
~% 
Atovaquone CAS#:95233-18-4 |
| Literature: Tetrahedron Letters, , vol. 39, # 42 p. 7629 - 7632 |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
CYP2C19*17 increases clopidogrel-mediated platelet inhibition but does not alter the pharmacokinetics of the active metabolite of clopidogrel.
Clin. Exp. Pharmacol. Physiol. 41(11) , 870-8, (2014) The aim of the present study was to determine the impact of CYP2C19*17 on the pharmacokinetics and pharmacodynamics of the active metabolite of clopidogrel and the pharmacokinetics of proguanil. Thus,... |
|
|
A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity.
Sci. Transl. Med. 7 , 290ra89, (2015) Currently, no approved therapeutics exist to treat or prevent infections induced by Ebola viruses, and recent events have demonstrated an urgent need for rapid discovery of new treatments. Repurposing... |
|
|
Atovaquone and quinine anti-malarials inhibit ATP binding cassette transporter activity.
Malaria Journal 13 , 359, (2014) Therapeutic blood plasma concentrations of anti-malarial drugs are essential for successful treatment. Pharmacokinetics of pharmaceutical compounds are dependent of adsorption, distribution, metabolis... |
| 2-Propanol,1-[2-(4-methyl-1,3-dioxolan-2-yl)ethoxy] |
| 2-[trans-4-(4'-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone |
| 1,2-Naphthalenedione, 3-[4-(4-chlorophenyl)cyclohexyl]-4-hydroxy- |
| MFCD00889188 |
| 3-(4-(4-chlorophenyl)cyclohexyl)-4-hydroxy-naphthalene-1,2-dione |
| trans-2-[2-(2-hydroxypropoxy)-ethyl]-4-methyl-1,3-dioxolane |
| Atovaquone |
| trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone |
| 3-[4-(4-Chlorophenyl)cyclohexyl]-4-hydroxy-1,2-naphthalenedione |
| 2-(trans-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthalenedione |
| (trans-2-hydroxy-3-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone) |
![cis/trans-1a-(4-(4-chlorophenyl)cyclohexyl)naphtho[2,3-b]oxyrene-2,7(1aH,7aH)-dione structure](https://image.chemsrc.com/caspic/010/1409957-21-6.png)