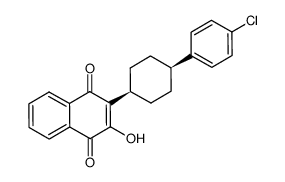
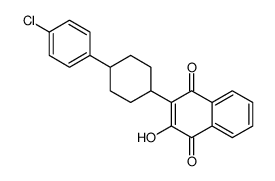
95233-18-4
| Name | atovaquone |
|---|---|
| Synonyms |
2-Propanol,1-[2-(4-methyl-1,3-dioxolan-2-yl)ethoxy]
2-[trans-4-(4'-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone 1,2-Naphthalenedione, 3-[4-(4-chlorophenyl)cyclohexyl]-4-hydroxy- MFCD00889188 3-(4-(4-chlorophenyl)cyclohexyl)-4-hydroxy-naphthalene-1,2-dione trans-2-[2-(2-hydroxypropoxy)-ethyl]-4-methyl-1,3-dioxolane Atovaquone trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone 3-[4-(4-Chlorophenyl)cyclohexyl]-4-hydroxy-1,2-naphthalenedione 2-(trans-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthalenedione (trans-2-hydroxy-3-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone) |
| Description | Atovaquone is a medication used to treat or prevent for pneumocystis pneumonia, toxoplasmosis, malaria, and babesia.Target: AntiparasiticAtovaquone (atavaquone) is a chemical compound that belongs to the class of naphthalenes. Atovaquone is a hydroxy-1,4-naphthoquinone, an analog of ubiquinone, with antipneumocystic activity [1]. Atovaquone is an anti-protozoal mitochondrial electron transport inhibitor; Antimalarial; Antipneumocystic, and has also been used to treat toxoplasmosis. It acts by inhibiting the cytochrome bc(1) complex via interactions with the Rieske iron-sulfur protein and cytochrome b in the ubiquinol oxidation pocket [2]. Atovaquone is a unique naphthoquinone with broad-spectrum antiprotozoal activity. It is effective for the treatment and prevention of Pneumocystis carinii pneumonia (PCP), it is effective in combination with proguanil for the treatment and prevention of malaria, and it is effective in combination with azithromycin for the treatment of babesiosis [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 542.2±50.0 °C at 760 mmHg |
| Melting Point | 216-2190C |
| Molecular Formula | C22H19ClO3 |
| Molecular Weight | 366.837 |
| Flash Point | 281.7±30.1 °C |
| Exact Mass | 366.102264 |
| PSA | 54.37000 |
| LogP | 5.86 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.653 |
| Storage condition | -20°C Freezer |
| Hazard Statements | H413 |
|---|---|
| Hazard Codes | N |
| Risk Phrases | 50/53 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | QJ5616500 |
| HS Code | 2942000000 |
|
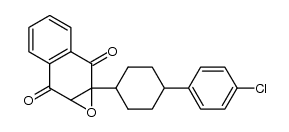
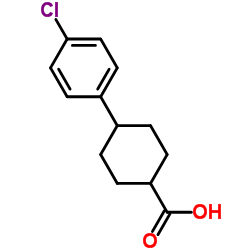
~85% 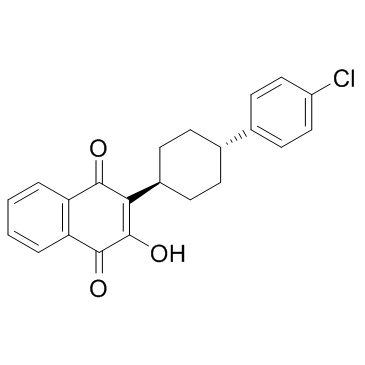
95233-18-4 |
| Literature: WO2010/1378 A1, ; Page/Page column 11 ; |
|
~78% 
95233-18-4 |
| Literature: US2010/137644 A1, ; Page/Page column 3-4 ; |
|
~93% 
95233-18-4 |
| Literature: Tetrahedron Letters, , vol. 39, # 42 p. 7629 - 7632 |
|
~% 
95233-18-4 |
| Literature: WO2012/153162 A1, ; Page/Page column 33 ; |
|
~% 
95233-18-4 |
| Literature: Tetrahedron Letters, , vol. 39, # 42 p. 7629 - 7632 |
|
~74% 
95233-18-4 |
| Literature: WO2013/14486 A1, ; Page/Page column 27; 28 ; |
|
~% 
95233-18-4 |
| Literature: Tetrahedron Letters, , vol. 39, # 42 p. 7629 - 7632 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |