A922500
A922500 structure
|
Common Name | A922500 | ||
|---|---|---|---|---|
| CAS Number | 959122-11-3 | Molecular Weight | 428.480 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 576.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C26H24N2O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 302.2±30.1 °C | |
Use of A922500A 922500 is a potent, selective, and orally bioavailable DGAT-1 inhibitor exhibiting IC50s of 9 and 22 nM against human and mouse DGAT-1, respectively. |
| Name | (1R,2R)-2-[4-[4-(phenylcarbamoylamino)phenyl]benzoyl]cyclopentane-1-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | A 922500 is a potent, selective, and orally bioavailable DGAT-1 inhibitor exhibiting IC50s of 9 and 22 nM against human and mouse DGAT-1, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 9 nM (human DGAT-1), 22 nM (mouse DGAT-1)[1] |
| In Vitro | A 922500 (A-922500) demonstrates excellent selectivity over other acyltransferases, including DGAT-2 (IC50=53 μM) and the phylogenetic family members acyl coenzyme A cholesterol acyltransferase-1 and -2 (IC50=296 μM) [1]. |
| In Vivo | DGAT-1 inhibitor A 922500 (A-922500) reduces serum triglyceride levels from baseline at all doses tested; however, this is only statistically significant at the 3 mg/kg dose, which lowers serum triglycerides by 53%. Similarly, the 3 mg/kg dose of A 922500 significantly reduces serum FFA concentrations by 55% and total cholesterol by 25%. DGAT-1 inhibition has no significant effect on body weight at any dose tested. Although A 922500 dpes not significantly affect LDL-cholesterol or HDL-cholesterol individually, the serum LDL/HDL-cholesterol ratio is significantly improved by A 922500 at 0.3 and 3 mg/kg. Similar to the dyslipidemic hamster, treatment with 3 mg/kg A 922500 significantly reduces serum triglyceride concentrations (39%). FFA levels significantly increase over the 14-day period in vehicle-treated animals. This increase is inhibited in a dose-dependent manner by A 922500 such that FFA concentrations are 32% lower after 14 days of treatment with the DGAT-1 inhibitor at 3 mg/kg, compared with the vehicle group (p < 0.05). HDL-cholesterol is significantly increased from baseline levels by A 922500 at 0.3 and 3 mg/kg; however, this is only significantly increased compared with vehicle at the 3 mg/kg dose. Body weight significantly increases over the 2-week period in vehicle-treated rats, and this is not affected by A 922500. LDL-cholesterol is significantly reduced in the vehicle treated group. DGAT-1 inhibition does not further reduce LDL-cholesterol and has no effect on total cholesterol[1]. |
| Animal Admin | Mice and Hamsters[1] Thirteen-week-old male Golden Syrian hamsters (n=40), initially weighing approximately 140 g, are used. Ten-week-old Male Zucker fatty rats (n=32), weighing between 270 and 330 g, are used. After collection of baseline lipid profiles, hyperlipidemic hamsters (n=10/group) and Zucker fatty rats (n=8/group) are administered vehicle [20:80 (v/v), polyethylene glycol/hydroxypropyl-β-cyclodextrin (10% w/v)] or DGAT-1 inhibitor A 922500 (A-922500) at 0.03, 0.3, and 3 mg/kg, once daily by oral gavage. The dosing volume is 5 mL/kg. Serum lipid profiles are then measured 3 h after the dose on day 7 and day 14. Hamsters continue to be fed a high-fat diet with 10% fructose in the drinking water throughout the treatment period. Zucker fatty rats remain on standard rodent diet throughout the study. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 576.1±50.0 °C at 760 mmHg |
| Molecular Formula | C26H24N2O4 |
| Molecular Weight | 428.480 |
| Flash Point | 302.2±30.1 °C |
| Exact Mass | 428.173615 |
| PSA | 95.50000 |
| LogP | 4.77 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.679 |
| Storage condition | Desiccate at +4°C |
|
~% 
A922500 CAS#:959122-11-3 |
| Literature: US2008/15227 A1, ; Page/Page column 17 ; US 20080015227 A1 |
|
~86% 
A922500 CAS#:959122-11-3 |
| Literature: Ravn, Matthew M.; Wagaw, Seble H.; Engstrom, Kenneth M.; Mei, Jianzhang; Kotecki, Brian; Souers, Andrew J.; Kym, Philip R.; Judd, Andrew S.; Zhao, Gang Organic Process Research and Development, 2010 , vol. 14, # 2 p. 417 - 424 |
|
~% 
A922500 CAS#:959122-11-3 |
| Literature: Organic Process Research and Development, , vol. 14, # 2 p. 417 - 424 |
|
~% 
A922500 CAS#:959122-11-3 |
| Literature: Organic Process Research and Development, , vol. 14, # 2 p. 417 - 424 |
|
~% 
A922500 CAS#:959122-11-3 |
| Literature: Organic Process Research and Development, , vol. 14, # 2 p. 417 - 424 |
|
~% 
A922500 CAS#:959122-11-3 |
| Literature: Organic Process Research and Development, , vol. 14, # 2 p. 417 - 424 |
|
~% 
A922500 CAS#:959122-11-3 |
| Literature: Organic Process Research and Development, , vol. 14, # 2 p. 417 - 424 |
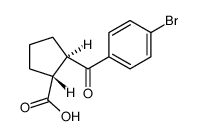
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| (1R,2R)-2-({4'-[(Phenylcarbamoyl)amino]-4-biphenylyl}carbonyl)cyclopentanecarboxylic acid |
| Cyclopentanecarboxylic acid, 2-[[4'-[[(phenylamino)carbonyl]amino][1,1'-biphenyl]-4-yl]carbonyl]-, (1R,2R)- |
| cc-142 |
| (1R,2R)-2-({4'-[(Phenylcarbamoyl)amino]biphenyl-4-yl}carbonyl)cyclopentanecarboxylic acid |
| A 922500 |
| DGAT-1 inhibitor |
![Methyl (1R,2R)-2-({4'-[(phenylcarbamoyl)amino]-4-biphenylyl}carbo nyl)cyclopentanecarboxylate structure](https://image.chemsrc.com/caspic/093/959122-10-2.png)
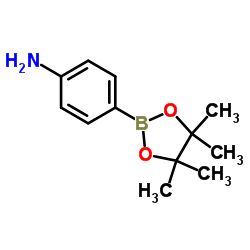
![Urea, N-phenyl-N'-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]- structure](https://image.chemsrc.com/caspic/170/819056-67-2.png)