CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
YV7875000
-
CHEMICAL NAME :
-
Valeric acid, 2-propyl-
-
CAS REGISTRY NUMBER :
-
99-66-1
-
BEILSTEIN REFERENCE NO. :
-
1750447
-
LAST UPDATED :
-
199712
-
DATA ITEMS CITED :
-
43
-
MOLECULAR FORMULA :
-
C8-H16-O2
-
MOLECULAR WEIGHT :
-
144.24
-
WISWESSER LINE NOTATION :
-
QVY3&3
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
375 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - pulse rate increase, without fall in BP Vascular - BP lowering not characterized in autonomic section Lungs, Thorax, or Respiration - cyanosis
-
REFERENCE :
-
CCMDC7 Critical Care Medicine. (Williams & Wilkins, 428 E. Preston Street, Baltimore, MD 21202) V.1- 1973- Volume(issue)/page/year: 21,299,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
412 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - coma Cardiac - pulse rate increase, without fall in BP Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
JATOD3 Journal of Analytical Toxicology. (Preston Pub. Inc., POB 48312, Niles, IL 60648) V.1- 1977- Volume(issue)/page/year: 20,55,1996
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
10500 mg/kg/30W-I
-
TOXIC EFFECTS :
-
Gastrointestinal - changes in structure or function of endocrine pancreas Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
AJDCAI American Journal of Diseases of Children. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1-80(3), 1911-50; V.100- 1960- Volume(issue)/page/year: 138,912,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
21 mg/kg/2D-I
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Nutritional and Gross Metabolic - other changes
-
REFERENCE :
-
NEURAI Neurology. (Modern Medicine Pub., Inc., 1 E. First St., Duluth, MN 55802) V.1- 1951- Volume(issue)/page/year: 37,886,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
1800 mg/kg/60D
-
TOXIC EFFECTS :
-
Gastrointestinal - changes in structure or function of endocrine pancreas Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 1,1196,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
13333 ug/kg/D-I
-
TOXIC EFFECTS :
-
Behavioral - sleep
-
REFERENCE :
-
NEJMAG New England Journal of Medicine. (Massachusetts Medical Soc., 10 Shattuck St., Boston, MA 02115) V.198- 1928- Volume(issue)/page/year: 301,435,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
670 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
FCTXAV Food and Cosmetics Toxicology. (London, UK) V.1-19, 1963-81. For publisher information, see FCTOD7. Volume(issue)/page/year: 2,327,1964
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1098 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
EPXXDW European Patent Application. (U.S. Patent and Trademark Office, Foreign Patents, Washington, DC 20231) Volume(issue)/page/year: #78785
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
470 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CHTPBA Chimica Therapeutica. (Paris, France) V.1-8, 1965-73. For publisher information, see EJMCA5. Volume(issue)/page/year: 3,430,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
860 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85GNAW "Pharmacodynamie de l'Acide Dipropylacetique (ou Propyl-2-Pentanoique) et de ses Amides," Carraz, G., Imprimerie Eymond, 1968 Volume(issue)/page/year: -,38,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1200 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85GNAW "Pharmacodynamie de l'Acide Dipropylacetique (ou Propyl-2-Pentanoique) et de ses Amides," Carraz, G., Imprimerie Eymond, 1968 Volume(issue)/page/year: -,39,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
824 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85GNAW "Pharmacodynamie de l'Acide Dipropylacetique (ou Propyl-2-Pentanoique) et de ses Amides," Carraz, G., Imprimerie Eymond, 1968 Volume(issue)/page/year: -,39,1968 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5480 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
REFERENCE :
-
AEPEDI Anales Espanoles de Pediatria. (Asociacion Espanola de Pediatria, Apartado 1176, 28028 Madrid, Spain) V.1- 1968- Volume(issue)/page/year: 39,19,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
720 mg/kg
-
SEX/DURATION :
-
female 1-90 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
CPEDAM Clinical Pediatrics (Philadelphia). (Lippincott/Harper, Journal Fulfillment Dept., 2350 Virginia Ave., Hagerstown, MD 21740) V.1- 1962- Volume(issue)/page/year: 22,336,1983
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
6525 mg/kg
-
SEX/DURATION :
-
female 26 week(s) pre-mating female 1-36 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - biochemical and metabolic Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
CPEDAM Clinical Pediatrics (Philadelphia). (Lippincott/Harper, Journal Fulfillment Dept., 2350 Virginia Ave., Hagerstown, MD 21740) V.1- 1962- Volume(issue)/page/year: 23,352,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
126 gm/kg
-
SEX/DURATION :
-
female 1-18 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
FDTHES Fetal Diagnosis and Therapy (Basel). (S. Karger, Postfach. CH-4009 Basel, Switzerland) V.5- 1990- Volume(issue)/page/year: 9,155,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
2394 mg/kg
-
SEX/DURATION :
-
female 1-36 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - Apgar score (human only)
-
REFERENCE :
-
JPETAB Journal of Pharmacology and Experimental Therapeutics. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1909/10- Volume(issue)/page/year: 219,768,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
2047 mg/kg
-
SEX/DURATION :
-
female 27-40 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
JPETAB Journal of Pharmacology and Experimental Therapeutics. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1909/10- Volume(issue)/page/year: 219,768,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
5320 mg/kg
-
SEX/DURATION :
-
female 2-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
JOPDAB Journal of Pediatrics. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63141) V.1- 1932- Volume(issue)/page/year: 97,332,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
5320 mg/kg
-
SEX/DURATION :
-
female 2-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
REFERENCE :
-
JOPDAB Journal of Pediatrics. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63141) V.1- 1932- Volume(issue)/page/year: 97,332,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
5320 mg/kg
-
SEX/DURATION :
-
female 2-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
REFERENCE :
-
JOPDAB Journal of Pediatrics. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63141) V.1- 1932- Volume(issue)/page/year: 97,332,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 45,603,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
34 gm/kg
-
SEX/DURATION :
-
male 60 day(s) pre-mating female 2 week(s) pre-mating - 1 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,58,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2400 mg/kg
-
SEX/DURATION :
-
female 7-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
NETEEC Neurotoxicology and Teratology. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.9- 1987- Volume(issue)/page/year: 13,471,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
6 gm/kg
-
SEX/DURATION :
-
female 8-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 35,28A,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
660 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - cytological changes (including somatic cell genetic material) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
ARTODN Archives of Toxicology. (Springer-Verlag, Heidelberger Pl. 3, D-1000 Berlin 33, Fed. Rep. Ger.) V.32- 1974- Volume(issue)/page/year: 64,545,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1500 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,58,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
432 mg/kg
-
SEX/DURATION :
-
female 11-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
TCMUD8 Teratogenesis, Carcinogenesis, and Mutagenesis. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1980- Volume(issue)/page/year: 1,367,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
432 mg/kg
-
SEX/DURATION :
-
female 8-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
TCMUD8 Teratogenesis, Carcinogenesis, and Mutagenesis. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1980- Volume(issue)/page/year: 1,367,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
519 mg/kg
-
SEX/DURATION :
-
female 8-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
TCMUD8 Teratogenesis, Carcinogenesis, and Mutagenesis. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1980- Volume(issue)/page/year: 1,367,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
AJMGDA American Journal of Medical Genetics. (John Wiley & Sons Ltd., 605 Third Ave., New York, NY 10158) V.1- 1977- Volume(issue)/page/year: 70,303,1997
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2400 mg/kg
-
SEX/DURATION :
-
female 7-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 1,763,1983
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 35,29A,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
18 gm/kg
-
SEX/DURATION :
-
female 21-50 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 33,45A,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4550 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,58,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4550 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
REFERENCE :
-
PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,58,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1950 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,58,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
945 mg/kg
-
SEX/DURATION :
-
female 6-8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
36DLAV "Clinical and Pharmacological Aspects of Sodium Valproate (Epilim) in the Treatment of Epilepsy, Proceedings of a Symposium, Nottingham, UK, 1975," Legg, N.J., et al., eds., Kent, UK, MCS Consultants, 1976 Volume(issue)/page/year: -,105,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 29(2),47A,1984 *** REVIEWS *** TOXICOLOGY REVIEW JOPDAB Journal of Pediatrics. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63141) V.1- 1932- Volume(issue)/page/year: 97,333,1980 TOXICOLOGY REVIEW PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,58,1979 TOXICOLOGY REVIEW APJUA8 Acta Pharmaceutica Jugoslavica. (FDH, Masarykova 2, 41000 Zagreb, Yugoslavia) V.1-41, 1951-1991. Volume(issue)/page/year: 41,79,1991 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X3895 No. of Facilities: 245 (estimated) No. of Industries: 4 No. of Occupations: 6 No. of Employees: 5848 (estimated) No. of Female Employees: 3255 (estimated)
|




 CAS#:1636-27-7
CAS#:1636-27-7 CAS#:13310-75-3
CAS#:13310-75-3 CAS#:76002-02-3
CAS#:76002-02-3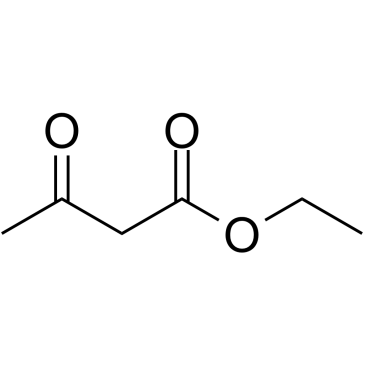 CAS#:141-97-9
CAS#:141-97-9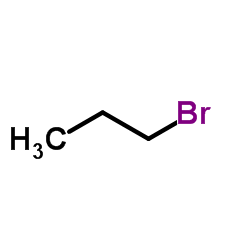 CAS#:106-94-5
CAS#:106-94-5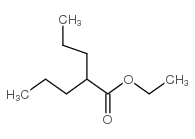 CAS#:17022-31-0
CAS#:17022-31-0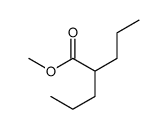 CAS#:22632-59-3
CAS#:22632-59-3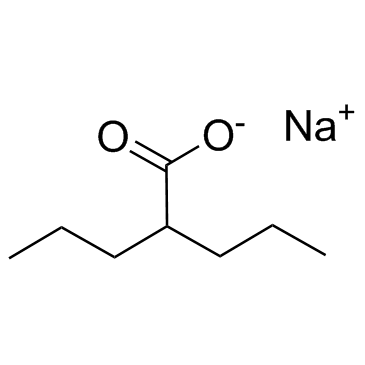 CAS#:1069-66-5
CAS#:1069-66-5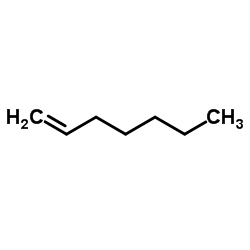 CAS#:592-76-7
CAS#:592-76-7 CAS#:201230-82-2
CAS#:201230-82-2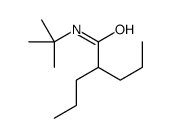 CAS#:2751-06-6
CAS#:2751-06-6 CAS#:2936-13-2
CAS#:2936-13-2 CAS#:2653-58-9
CAS#:2653-58-9 CAS#:2936-12-1
CAS#:2936-12-1![N-[2-(dimethylamino)ethyl]-2-propylpentanamide structure](https://image.chemsrc.com/caspic/176/2750-95-0.png) CAS#:2750-95-0
CAS#:2750-95-0 CAS#:2936-11-0
CAS#:2936-11-0 CAS#:99174-91-1
CAS#:99174-91-1 CAS#:58175-57-8
CAS#:58175-57-8
