6-氯嘌呤
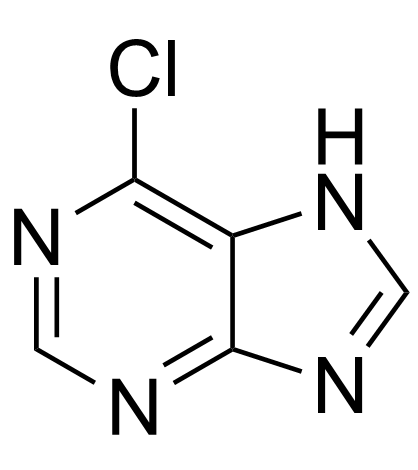
6-氯嘌呤结构式

|
常用名 | 6-氯嘌呤 | 英文名 | 6-chloropurine |
|---|---|---|---|---|
| CAS号 | 87-42-3 | 分子量 | 154.557 | |
| 密度 | 1.7±0.1 g/cm3 | 沸点 | 449.6±25.0 °C at 760 mmHg | |
| 分子式 | C5H3ClN4 | 熔点 | >300 °C (dec.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 258.2±8.8 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Synthesis of modified homo-N-nucleosides from the reactions of mesityl nitrile oxide with 9-allylpurines and their influence on lipid peroxidation and thrombin inhibition
Bioorg. Med. Chem. Lett. 19 , 6433-6, (2009) The synthesized compounds act as lipid peroxidation inhibitors and potent thrombin inhibitors. |
|
|
The synthesis of novel fluorescent purine analogues modified by azacrown ether at C6
Bioorg. Med. Chem. Lett. 20(10) , 3098-102, (2010) The synthesis and fluorescence properties of novel purine analogues linked azacrown ether at C6 position were investigated. These new purine analogues could be prepared from a series of 6-chloropurines and showed selective and efficient signaling behaviors to... |
|
|
Evidence for Watson-Crick and not Hoogsteen or wobble base pairing in the selection of nucleotides for insertion opposite pyrimidines and a thymine dimer by yeast DNA pol eta.
Biochemistry 44(12) , 4850-60, (2005) We have recently reported that pyrene nucleotide is preferentially inserted opposite an abasic site, the 3'-T of a thymine dimer, and most undamaged bases by yeast DNA polymerase eta (pol eta). Because pyrene is a nonpolar molecule with no H-bonding ability, ... |
|
|
Asymmetric synthesis of novel thioiso dideoxynucleosides with exocyclic methylene as potential antiviral agents.
J. Org. Chem. 69(9) , 3208-11, (2004) Novel thioiso pyrimidine and purine nucleosides substituted with exocyclic methylene have been synthesized, starting from D-xylose. The glycosyl donor 14 was synthesized from D-xylose, using cyclization of dimesylate 10 with sodium sulfide as a key step. Cycl... |
|
|
Synthesis and biological evaluation of nucleoside analogues having 6-chloropurine as anti-SARS-CoV agents.
Bioorg. Med. Chem. Lett. 17 , 2470-3, (2007) Nucleoside analogues that have 6-chloropurine as the nucleobase were synthesized and evaluated for anti-SARS-CoV activity by plaque reduction and yield reduction assays in order to develop novel anti-SARS-CoV agents. Among these analogues, two compounds, name... |
|
|
Microwave-assisted amination of a chloropurine derivative in the synthesis of acyclic nucleoside analogues.
Molecules 10(2) , 508-15, (2005) An efficient protocol for the amination of 6-chloropurine derivatives through nucleophilic aromatic substitution under microwave irradiation was developed and applied to the synthesis in two steps of a series of new acyclic nucleosides (acyclovir analogues) s... |
|
|
Facile synthesis of 1',2'-cis-beta-pyranosyladenine nucleosides.
Carbohydr. Res. 342(17) , 2641-8, (2007) 1',2'-cis-beta-Glycosyladenine nucleosides, such as beta-altroside, beta-mannoside, and beta-idoside, were efficiently synthesized from the corresponding 1',2'-trans-beta-6-chloropurine derivatives, beta-glucoside, and beta-galactoside. Nucleophilic substitut... |
|
|
15N and 13C NMR chemical shifts of 6-(fluoro, chloro, bromo, and iodo)purine 2'-deoxynucleosides: measurements and calculations.
Magn. Reson. Chem. 48(1) , 61-7, (2010) The (15)N and (13)C chemical shifts of 6-(fluoro, chloro, bromo, and iodo)purine 2'-deoxynucleoside derivatives in deuterated chloroform were measured. The (15)N chemical shifts were determined by the (1)H-(15)N HMBC method, and complete (15)N chemical-shift ... |
|
|
Purification and preliminary characterization of (E)-3-(2,4-dioxo-6-methyl-5-pyrimidinyl)acrylic acid synthase, an enzyme involved in biosynthesis of the antitumor agent sparsomycin.
J. Bacteriol. 179(4) , 1385-92, (1997) Sparsomycin is an antitumor antibiotic produced by Streptomyces sparsogenes. Biosynthetic experiments have previously demonstrated that one component of sparsomycin is derived from L-tryptophan via the intermediacy of (E)-3-(4-oxo-6-methyl-5-pyrimidinyl)acryl... |
|
|
Synthesis of 6-aryloxy- and 6-arylalkoxy-2-chloropurines and their interactions with purine nucleoside phosphorylase from Escherichia coli.
Z. Naturforsch., C, J. Biosci. 54(12) , 1055-67, (1999) The phase transfer method was applied to perform the nucleophilic substitution of 2,6-dichloropurines by modified arylalkyl alcohol or phenols. Since under these conditions only the 6-halogen is exchanged, this method gives 2-chloro-6-aryloxy- and 2-chloro-6-... |